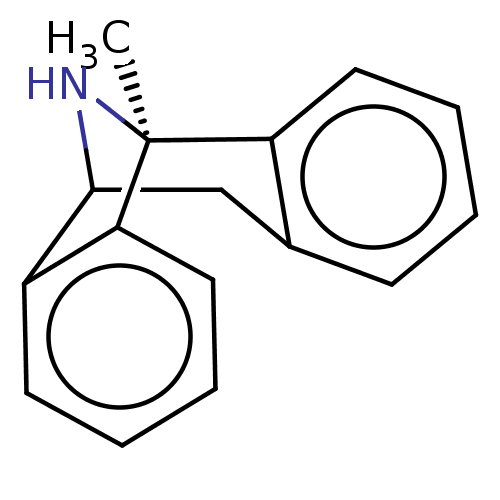
BDBM50000663 (+)-1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene::(+)-MK-801::(+)MK-801::(+/-) MK-8011-methyl-(9R,1R)-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene::(+/-)-1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene::(+/-)-MK801::(-)-1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene::(-)-MK801::(1S,9R)-1-methyl-16-azatetracyclo[7.6.1.0^{2,7}.0^{10,15}]hexadeca-2(7),3,5,10(15),11,13-hexaene::(1S,9R)-1-methyl-16-azatetracyclo[7.6.1.0^{2,7}.0^{10,15}]hexadeca-2,4,6,10(15),11,13-hexaene::(5S,10R)-(+)-5-methyl-10,11-dihydro-5Hdibenzo[a,d]cyclohepten-5,10-imine::(5S,10S)-(+)-5-methyl-10,11-dihydro-5Hdibenzo[a,d]cyclohepten-5,10-imine::(Dizocilpine)1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene::(MK-801)1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene::1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene::1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene(MK-801)::1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10,12,14-hexaene::1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2,4,6,10(15),11,13-hexaene::10,11-Dihydro-5-methyl-5H-dibenzo[a,d]cyclohepten-5,10-imine.(MK-801)::CHEMBL284237::MK-801::MK-801 (Dizocilpine)::MK-801,(+)::MK-801,(-)::US11944616, Compound Dizocilpine::dizocilpine
SMILES C[C@]12NC(Cc3ccccc13)c1ccccc21
InChI Key InChIKey=LBOJYSIDWZQNJS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 18 hits for monomerid = 50000663
Found 18 hits for monomerid = 50000663
Institut National De La Sante Et De La Recherche Medicale (Inserm
US Patent
Institut National De La Sante Et De La Recherche Medicale (Inserm
US Patent
Institut National De La Sante Et De La Recherche Medicale (Inserm
US Patent
Institut National De La Sante Et De La Recherche Medicale (Inserm
US Patent
Institut National De La Sante Et De La Recherche Medicale (Inserm
US Patent
Institut National De La Sante Et De La Recherche Medicale (Inserm
US Patent
Institut National De La Sante Et De La Recherche Medicale (Inserm
US Patent
Institut National De La Sante Et De La Recherche Medicale (Inserm
US Patent
Institut National De La Sante Et De La Recherche Medicale (Inserm
US Patent
Institut National De La Sante Et De La Recherche Medicale (Inserm
US Patent
Institut National De La Sante Et De La Recherche Medicale (Inserm
US Patent
Institut National De La Sante Et De La Recherche Medicale (Inserm
US Patent
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL