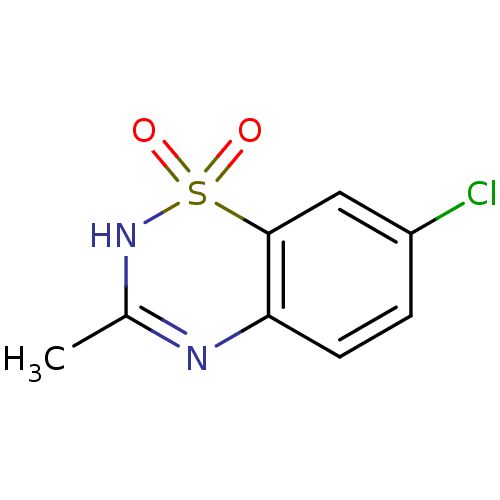
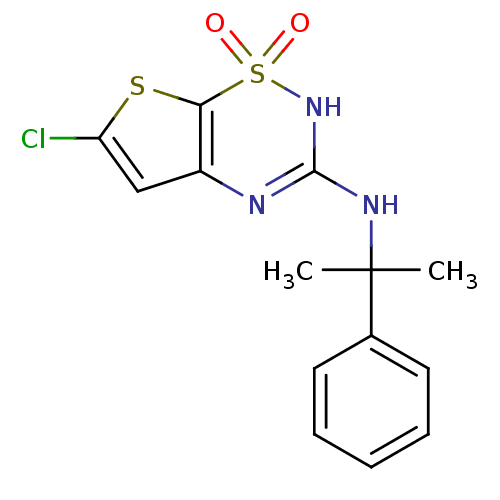
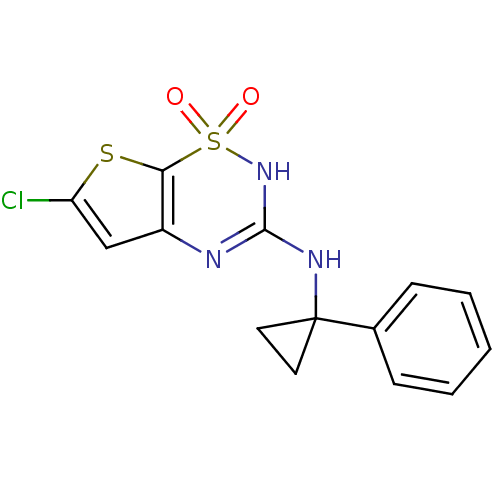
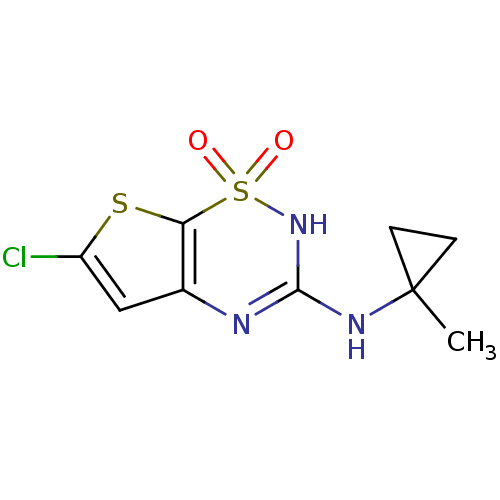
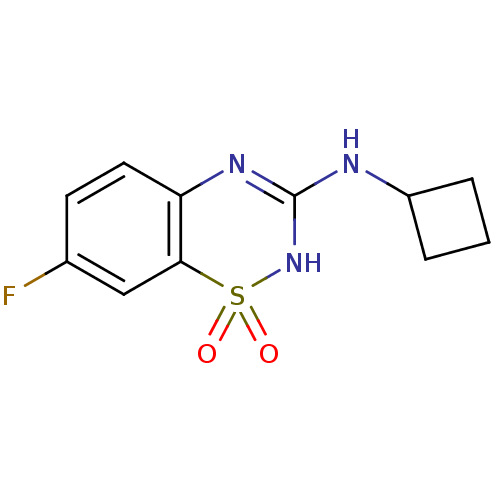
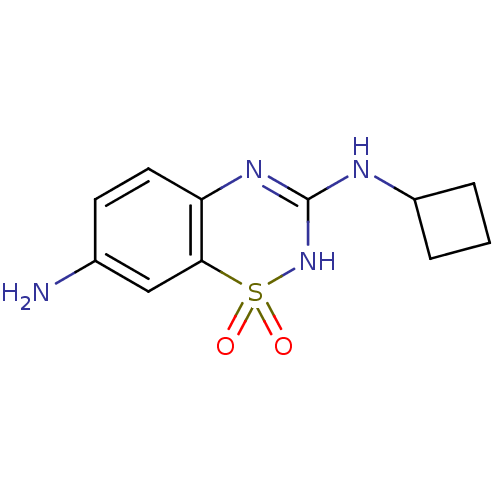
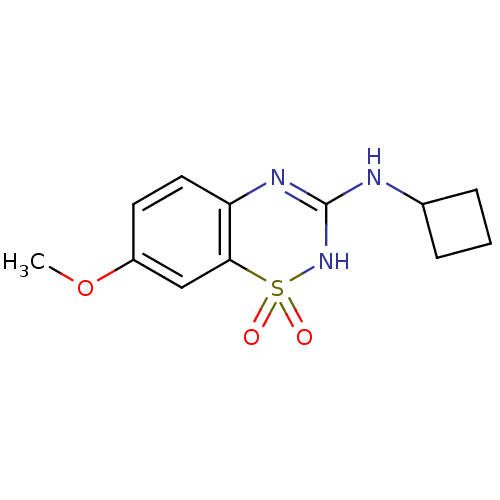
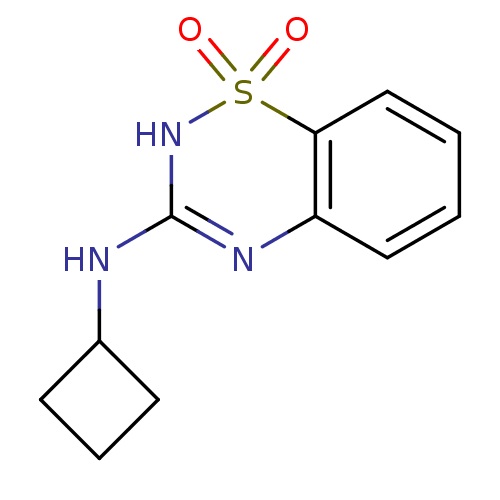
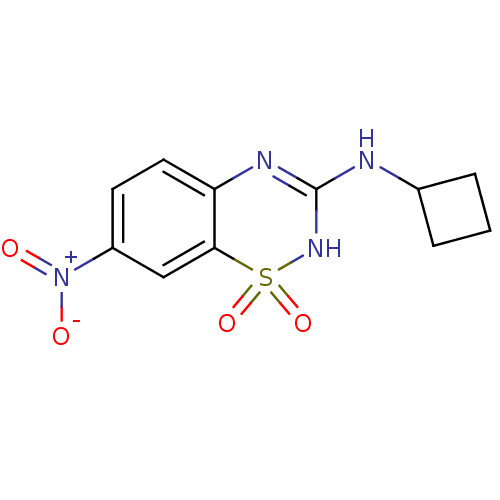
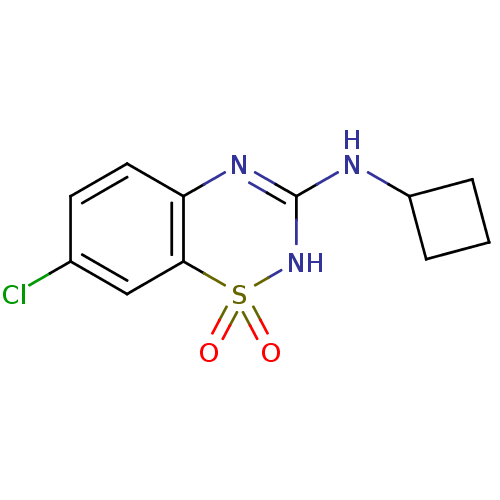
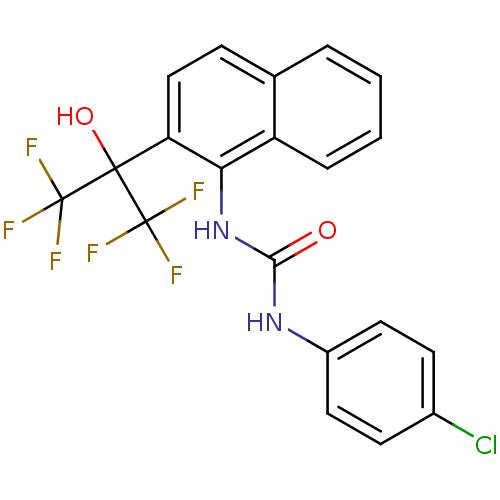
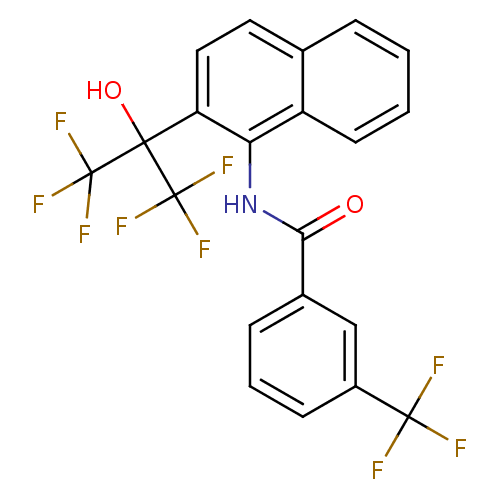
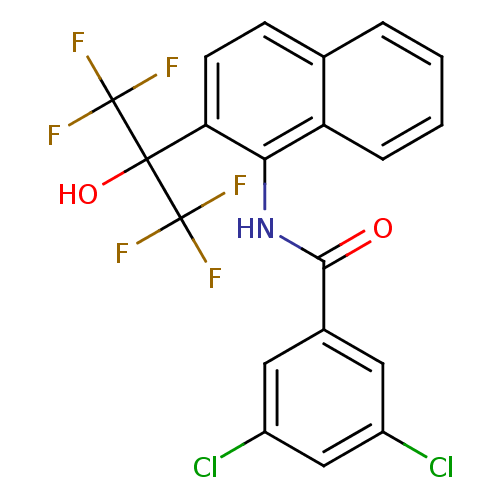
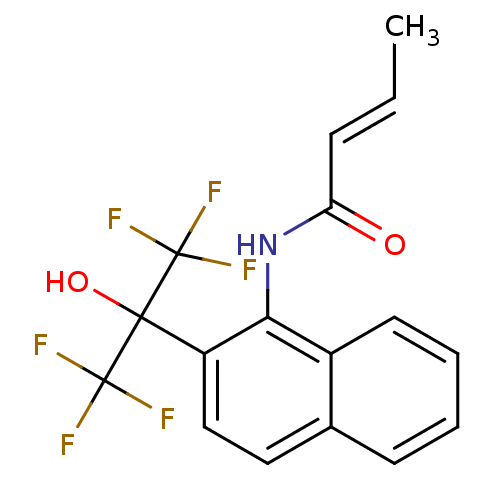
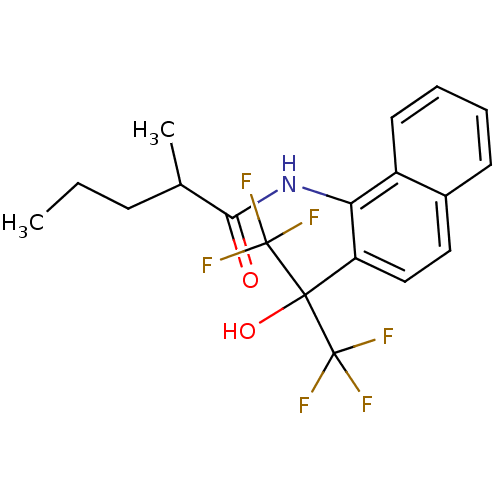
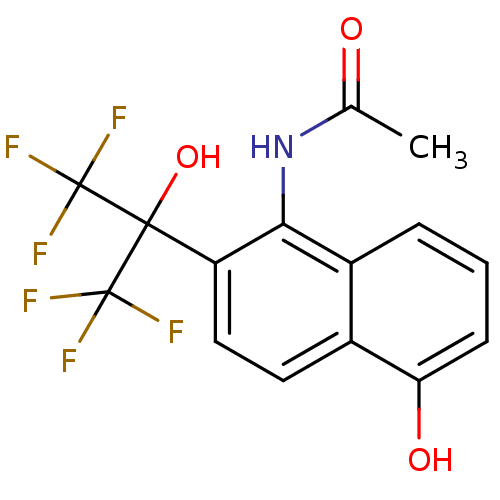
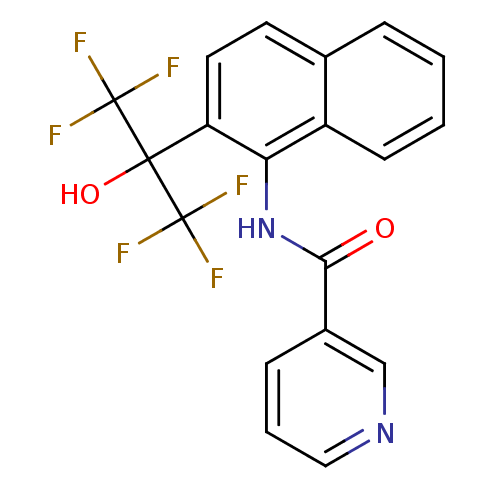
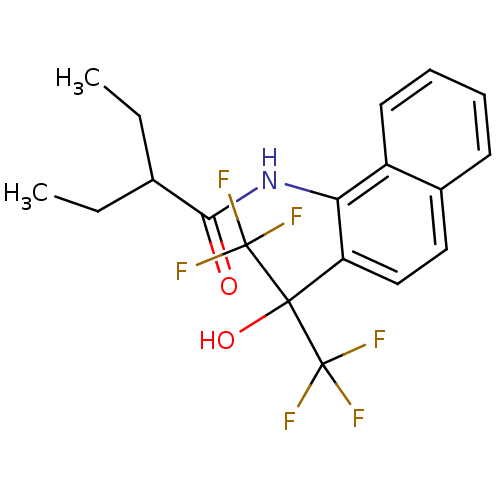
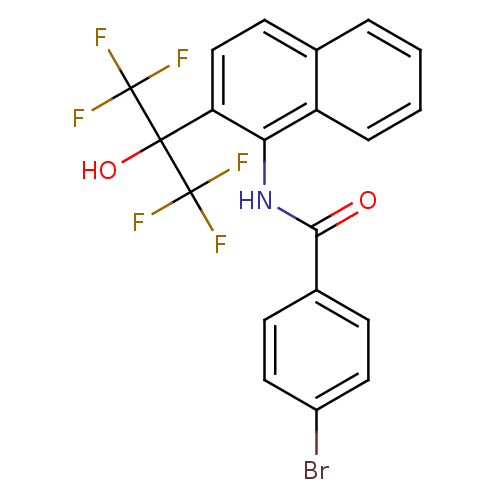
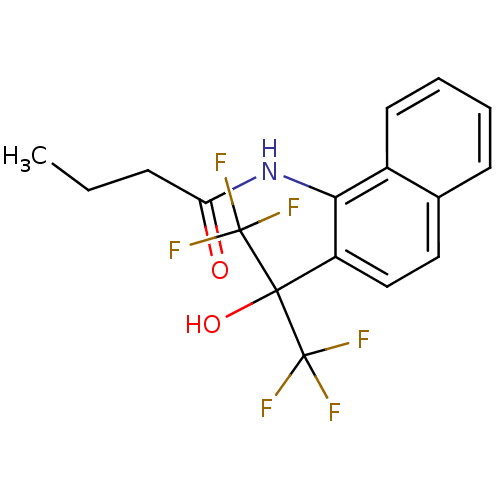
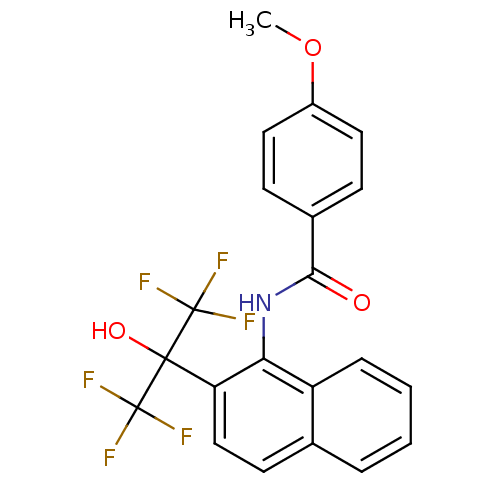
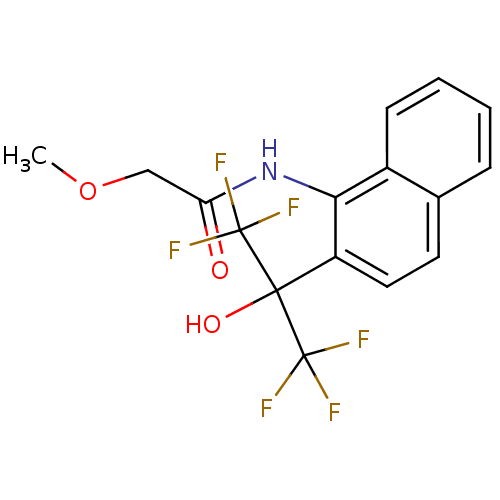
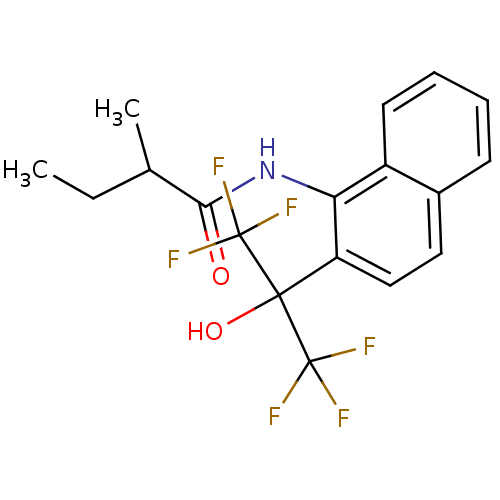
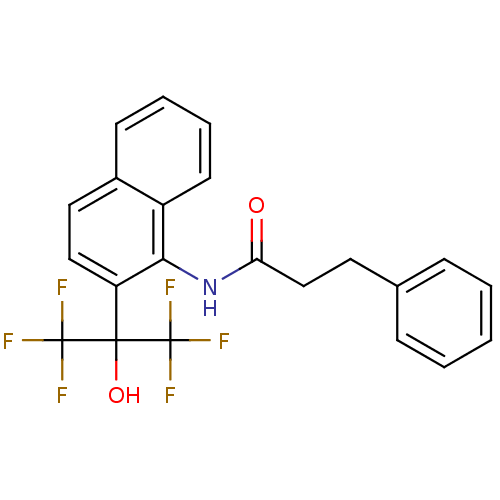
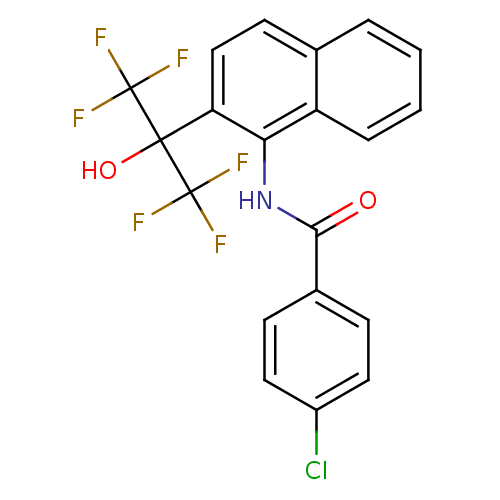
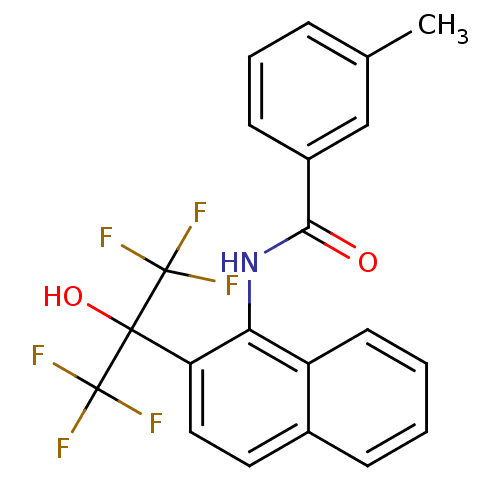
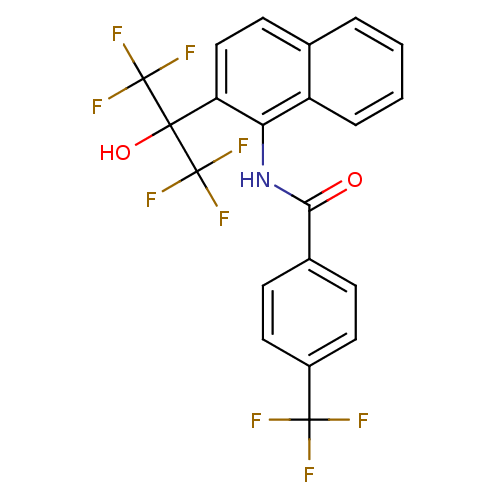
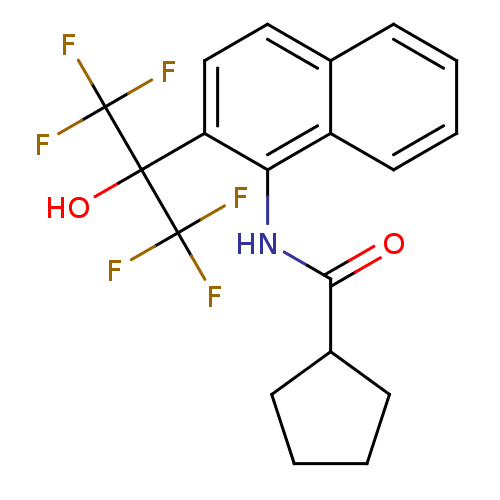
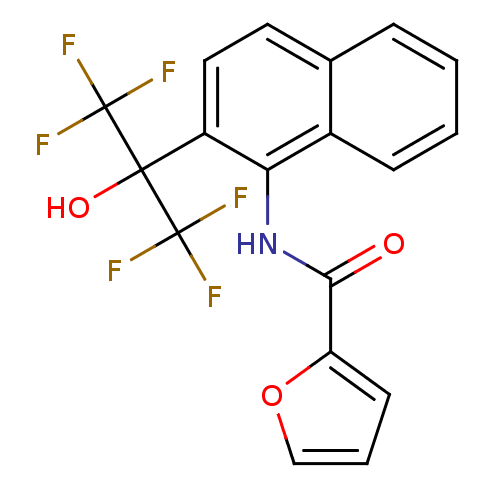
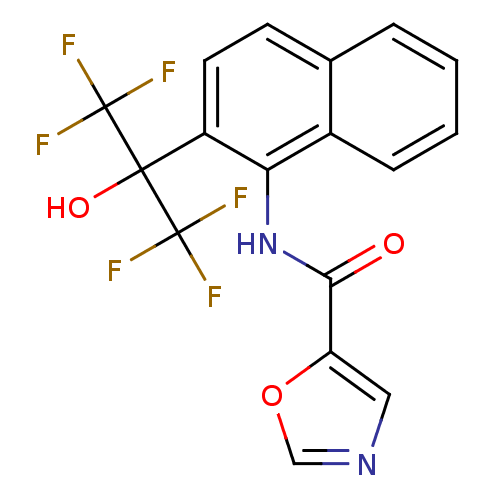
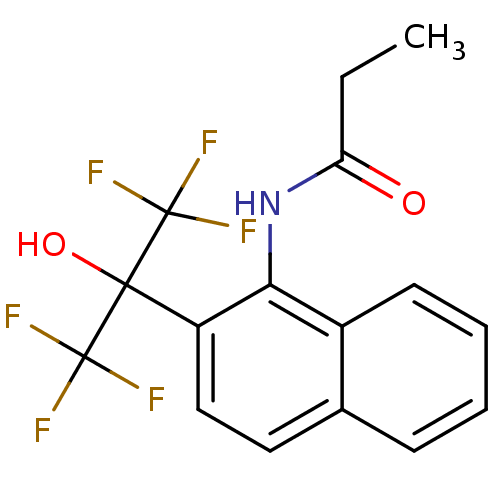
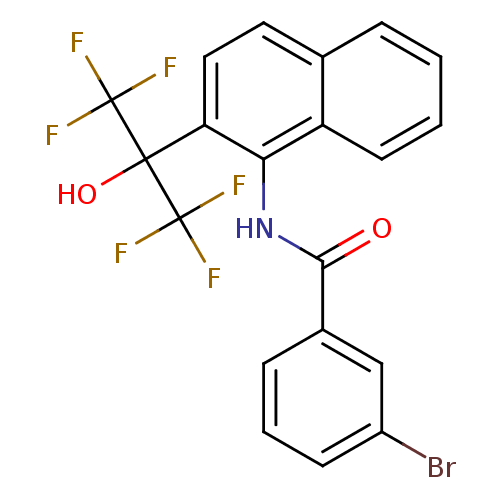
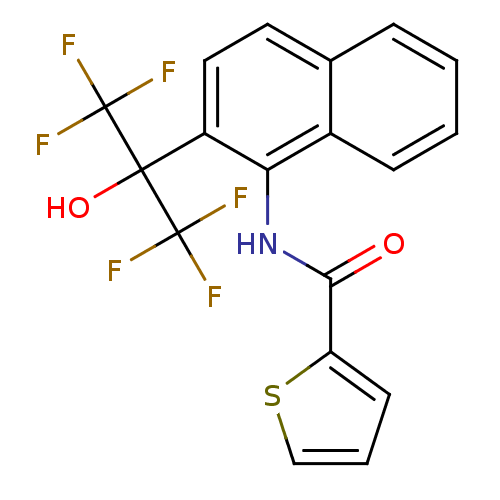
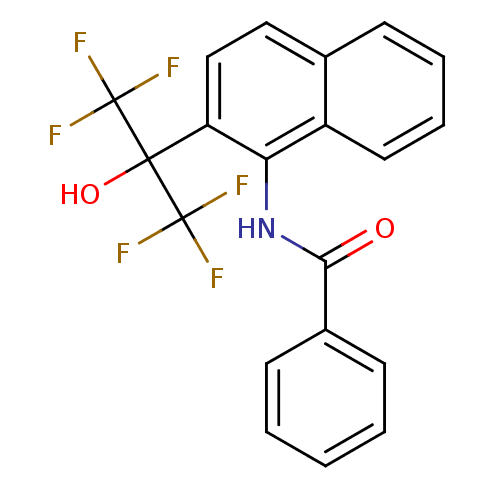
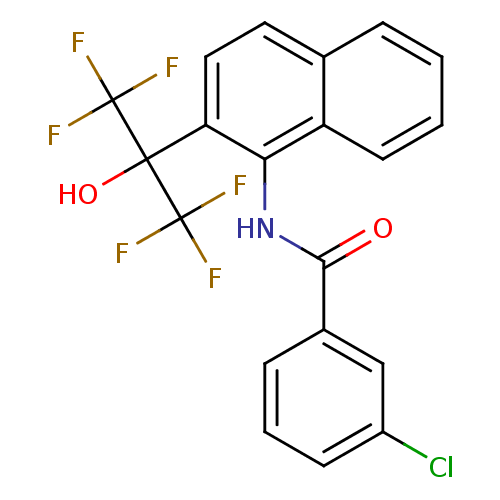
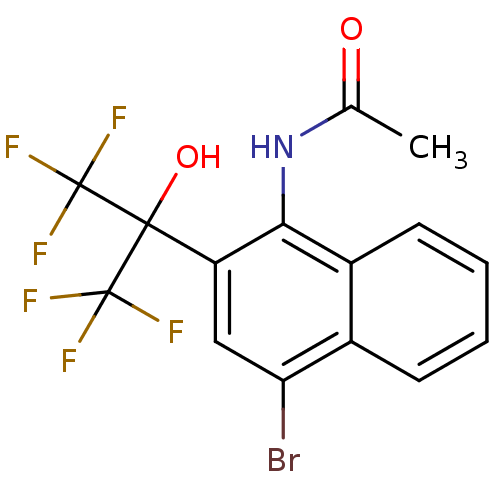
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 530nMAssay Description:Repolarization of HEK293 cells expressing Kir6.2/SUR1 KATP channelsMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 3.30E+4nMAssay Description:Repolarization of HEK293 cells expressing Kir6.2/SUR1 KATP channelsMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 180nMAssay Description:Repolarization of HEK293 cells expressing Kir6.2/SUR1 KATP channelsMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 810nMAssay Description:Repolarization of HEK293 cells expressing Kir6.2/SUR1 KATP channelsMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 190nMAssay Description:Repolarization of HEK293 cells expressing Kir6.2/SUR1 KATP channelsMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 160nMAssay Description:Repolarization of HEK293 cells expressing Kir6.2/SUR1 KATP channelsMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 190nMAssay Description:Activity at Kir6.2/SUR1 KATP channels expressed in HEK293 cells assessed as repolarization of tolbutamide-induced membrane depolarizationMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 3.10E+4nMAssay Description:Activity at Kir6.2/SUR1 KATP channels expressed in HEK293 cells assessed as activation of K+ currentsMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 356nMAssay Description:Inhibition of Beta-cell KATP channelMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 3.20E+4nMAssay Description:Negative log EC50 for Beta-cell KATP channelMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 4.00E+3nMAssay Description:Negative log EC50 for Beta-cell KATP channelMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 2.00E+3nMAssay Description:Negative log EC50 for Beta-cell KATP channelMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 530nMAssay Description:Inhibition of Beta-cell KATP channelMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 3.10E+4nMAssay Description:Inhibition of Beta-cell KATP channelMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 3.87E+3nMAssay Description:Inhibition of Beta-cell KATP channelMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 8.80E+3nMAssay Description:Inhibition of Beta-cell KATP channelMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 1.96E+3nMAssay Description:Inhibition of Beta-cell KATP channelMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 196nMAssay Description:Inhibition of Beta-cell KATP channelMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 200nMAssay Description:Negative log EC50 for Beta-cell KATP channelMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Human)
Novo Nordisk Research and Development
Curated by ChEMBL
Novo Nordisk Research and Development
Curated by ChEMBL
Affinity DataEC50: 400nMAssay Description:Negative log EC50 for Beta-cell KATP channelMore data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 276nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 84nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 306nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 72nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 137nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 60nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 4.34E+3nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 47nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 131nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 417nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 213nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 1.77E+5nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 1.75E+3nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 570nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 23nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 160nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 495nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 44nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 9nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 24nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 15nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 80nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 12nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 44nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 98nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 5.31E+3nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 751nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 11(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 5.55E+3nMAssay Description:Potassium channel opening activity in vitro using LtK cells transfected with Kir6.2/SUR2B exon 17More data for this Ligand-Target Pair