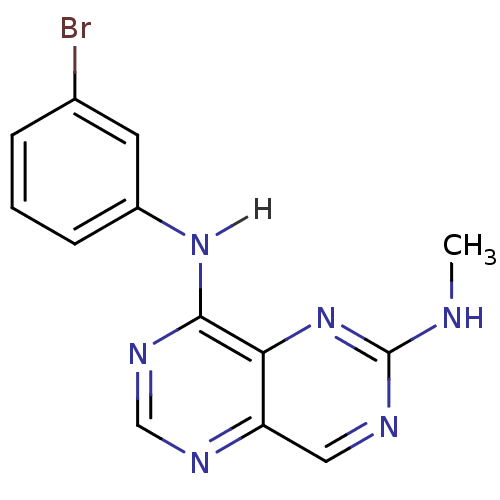
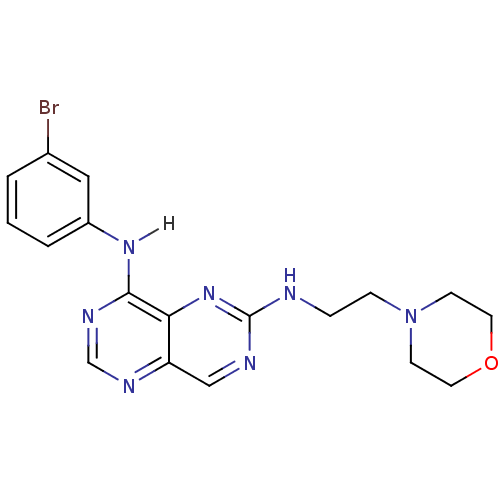
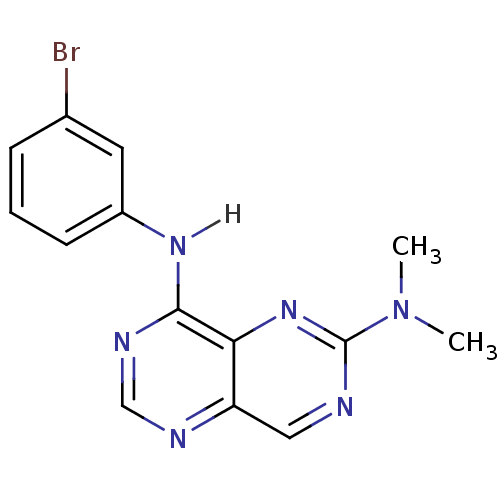
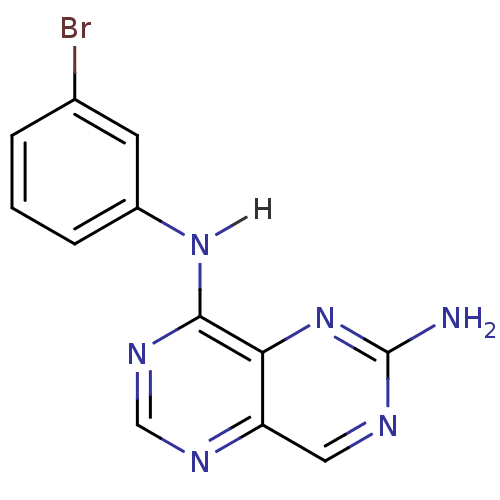
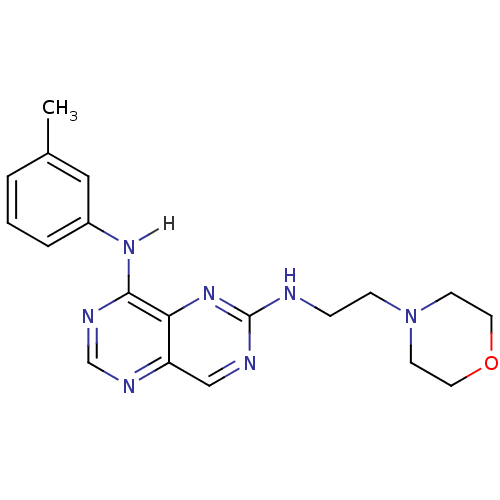
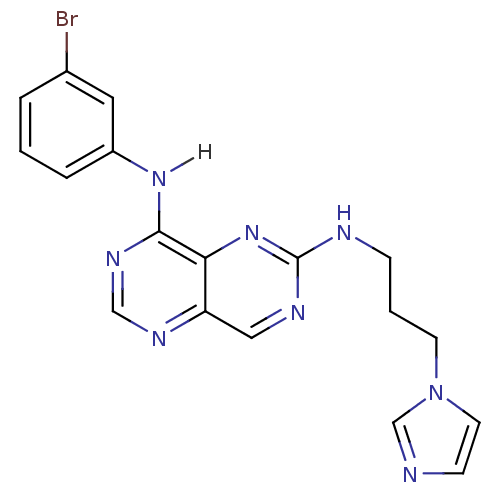
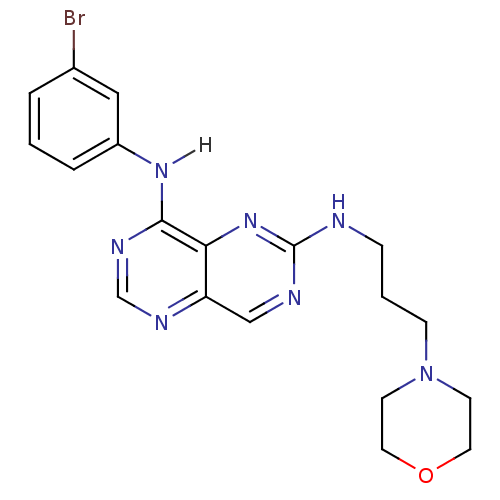
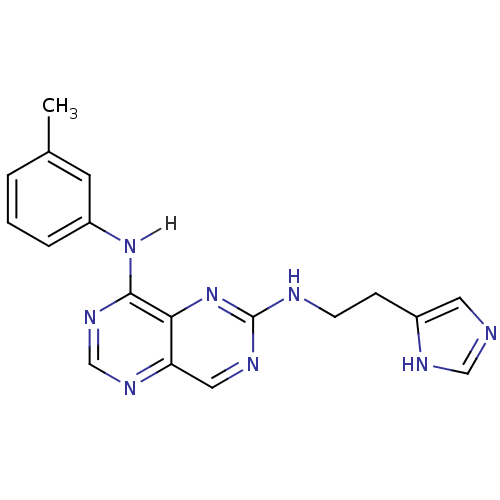
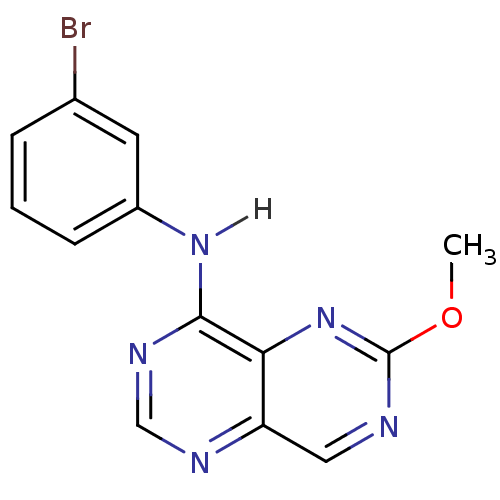
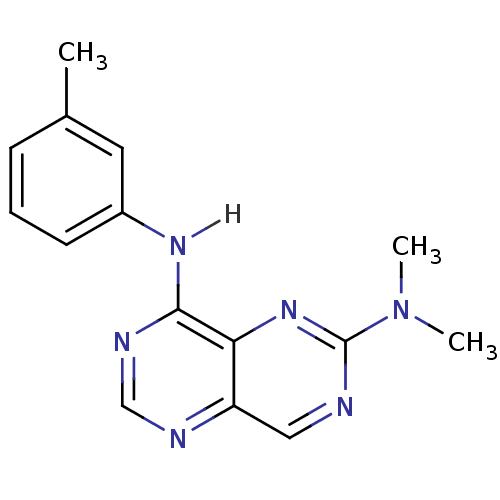
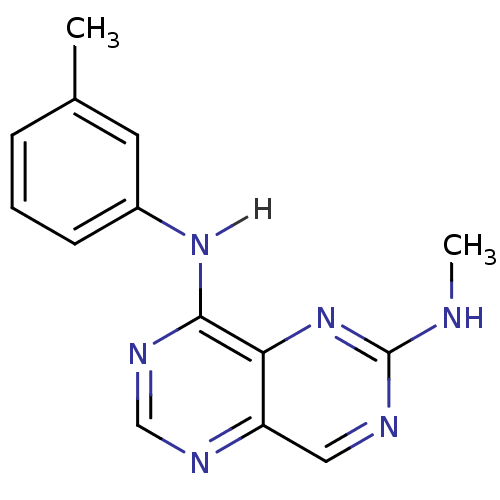
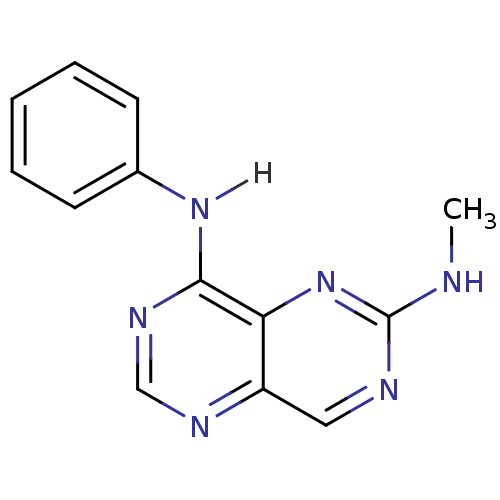
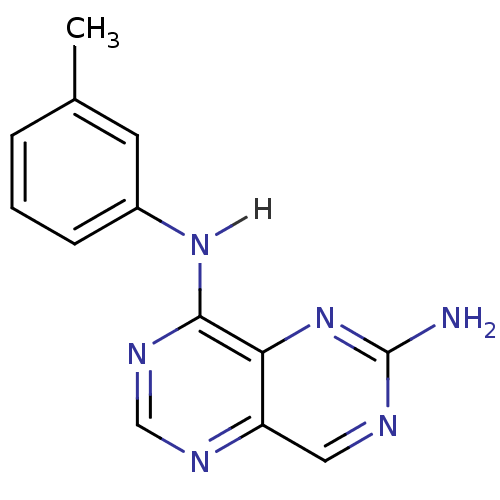
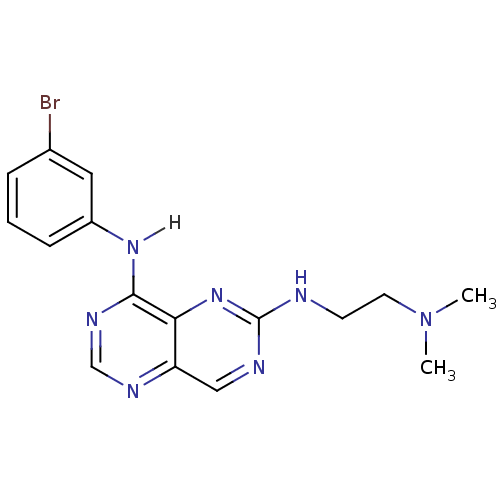
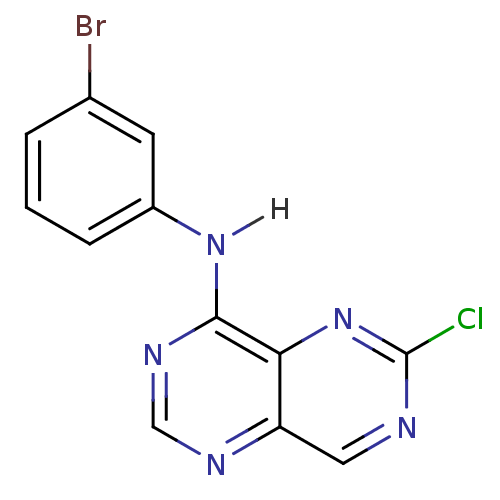
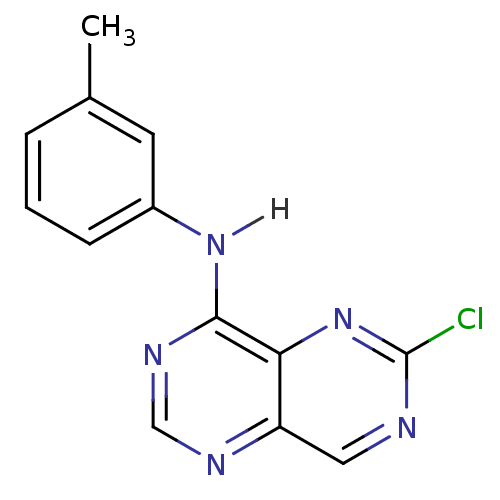
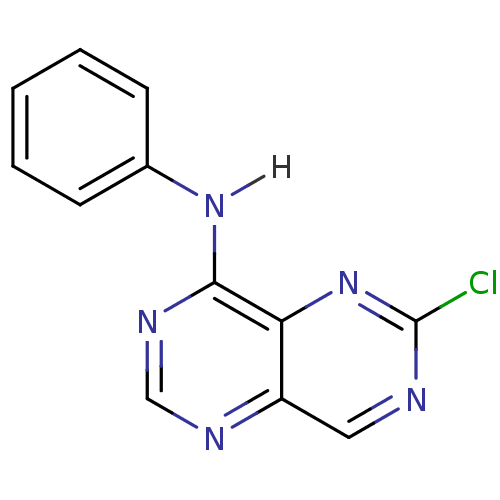
Report error Found 18 Enz. Inhib. hit(s) with all data for entry = 551
Affinity DataIC50: 0.25nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.760nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.810nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.950nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 35nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 82nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 380nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 2.55E+3nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair