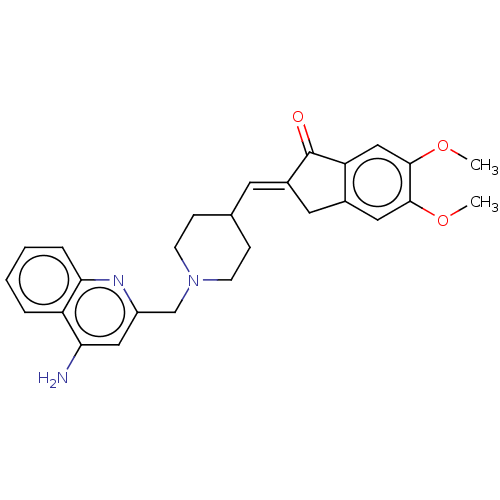
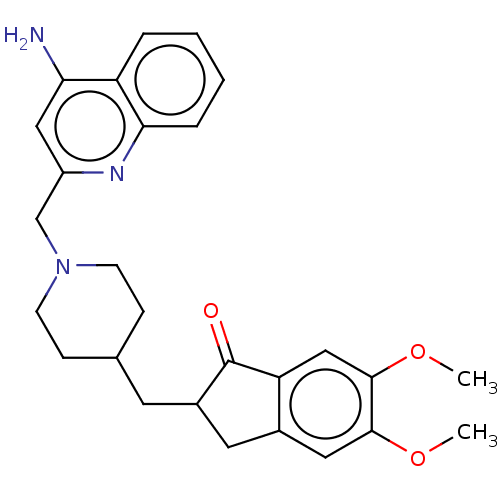
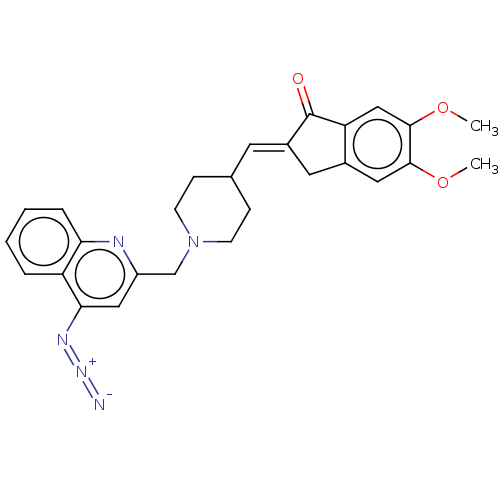
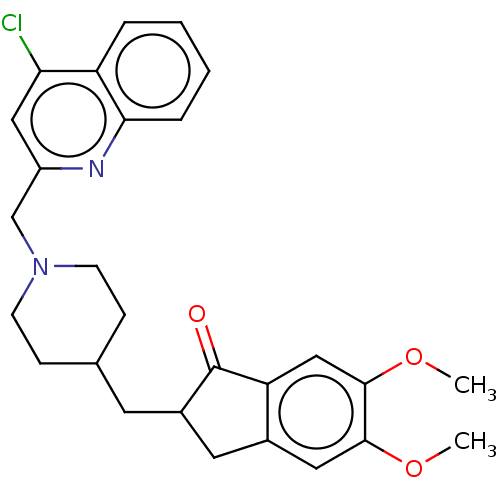
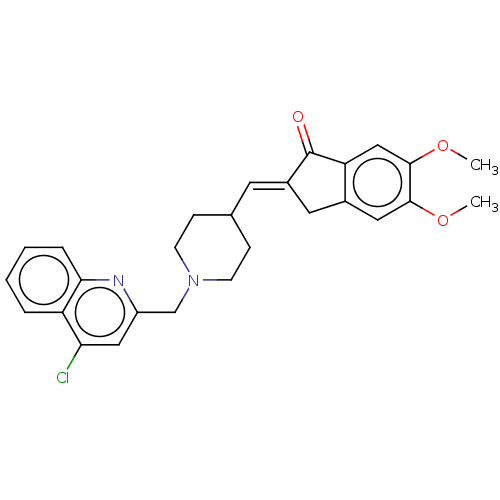
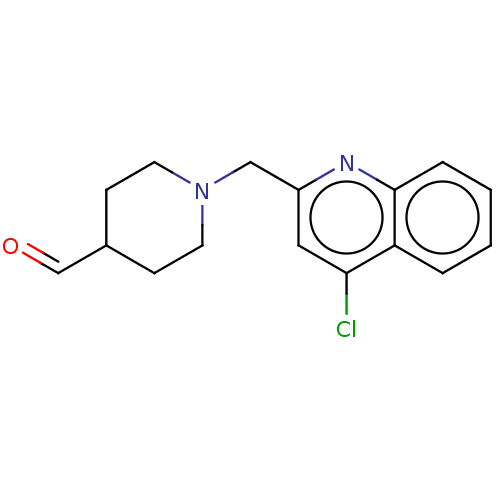
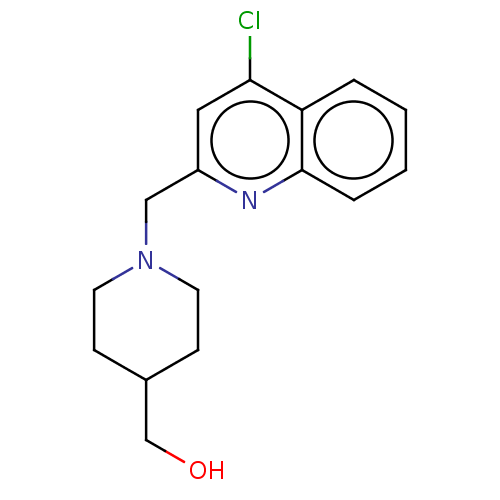
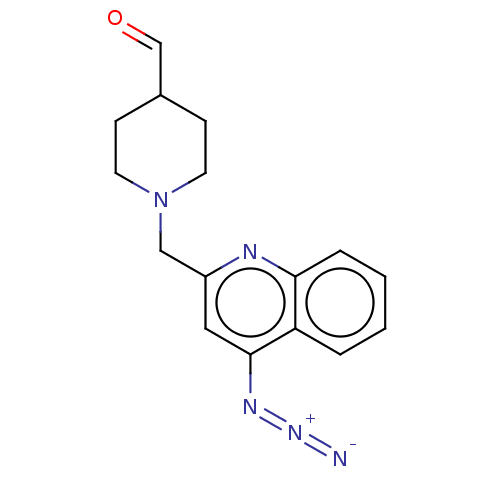
Report error Found 21 Enz. Inhib. hit(s) with all data for entry = 50001360
Affinity DataIC50: 0.270nMAssay Description:Inhibition of AChE in human erythrocytes acetylthiocholine iodide as substrate by Ellman's spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 230nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U...More data for this Ligand-Target Pair
Affinity DataIC50: 710nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U...More data for this Ligand-Target Pair
Affinity DataIC50: 3.69E+3nMAssay Description:Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 4.88E+3nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U...More data for this Ligand-Target Pair
Affinity DataIC50: 5.99E+3nMAssay Description:Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 6.54E+3nMAssay Description:Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 9.14E+3nMAssay Description:Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U...More data for this Ligand-Target Pair
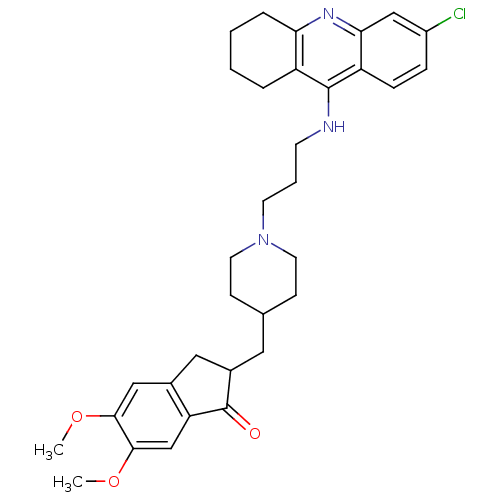
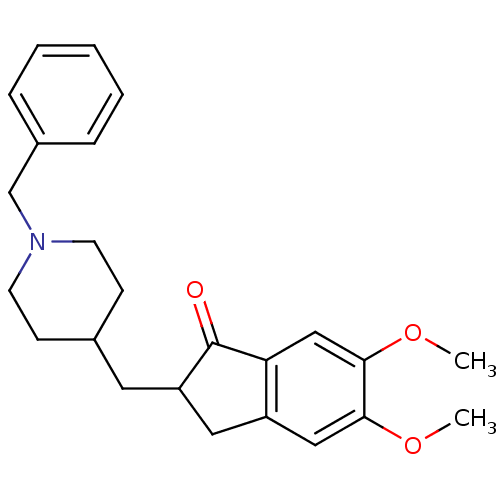
 3D Structure (crystal)
3D Structure (crystal)