Report error Found 37 Enz. Inhib. hit(s) with all data for entry = 50033938
Affinity DataEC50: 63.1nMAssay Description:Agonist activity at human S1P5 receptor expressed in CHO-K1 aequorin cells assessed as calcium accumulation of by luminescence assay in presence of c...More data for this Ligand-Target Pair
Affinity DataEC50: >3.16E+4nMAssay Description:Agonist activity at human S1P2 receptor expressed in yeast MMY24 assessed as conversion of FDGlu to fluorescein after 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: >3.16E+4nMAssay Description:Agonist activity at human S1P3 receptor expressed in RBL cells assessed as [35S]GTPgammaS binding after 3 hrs by radiometric assayMore data for this Ligand-Target Pair
Affinity DataEC50: >3.16E+4nMAssay Description:Agonist activity at human S1P3 receptor expressed in RBL cells assessed as [35S]GTPgammaS binding after 3 hrs by radiometric assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.51E+4nMAssay Description:Agonist activity at human S1P3 receptor expressed in RBL cells assessed as [35S]GTPgammaS binding after 3 hrs by radiometric assayMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+5nMAssay Description:Agonist activity at human S1P3 receptor expressed in RBL cells assessed as [35S]GTPgammaS binding after 3 hrs by radiometric assayMore data for this Ligand-Target Pair
Affinity DataEC50: 19.9nMAssay Description:Agonist activity at human S1P5 receptor expressed in CHO-K1 aequorin cells assessed as calcium accumulation of by luminescence assay in presence of c...More data for this Ligand-Target Pair
Affinity DataEC50: 1.58E+4nMAssay Description:Agonist activity at human S1P3 receptor expressed in RBL cells assessed as [35S]GTPgammaS binding after 3 hrs by radiometric assayMore data for this Ligand-Target Pair
Affinity DataEC50: >4.17E+4nMAssay Description:Agonist activity at human S1P4 expressed in CHO-K1 aequorin cells assessed as calcium accumulation of by luminescence assay in presence of cofactor c...More data for this Ligand-Target Pair
Affinity DataEC50: >3.31E+4nMAssay Description:Agonist activity at human S1P2 receptor expressed in yeast MMY24 assessed as conversion of FDGlu to fluorescein after 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 6.31E+3nMAssay Description:Agonist activity at human S1P3 receptor expressed in RBL cells assessed as [35S]GTPgammaS binding after 3 hrs by radiometric assayMore data for this Ligand-Target Pair
Affinity DataEC50: 200nMAssay Description:Agonist activity at human S1P4 expressed in CHO-K1 aequorin cells assessed as calcium accumulation of by luminescence assay in presence of cofactor c...More data for this Ligand-Target Pair
Affinity DataEC50: 162nMAssay Description:Agonist activity at human S1P5 receptor expressed in CHO-K1 aequorin cells assessed as calcium accumulation of by luminescence assay in presence of c...More data for this Ligand-Target Pair
Affinity DataEC50: 19.9nMAssay Description:Agonist activity at human S1P1 receptor expressed in CHO-K1 EDG1 cells expressing beta-arrestin 2 after 105 mins by chemi-luminescence assayMore data for this Ligand-Target Pair
Affinity DataEC50: 19.9nMAssay Description:Agonist activity at human S1P1 receptor expressed in CHO-K1 EDG1 cells expressing beta-arrestin 2 after 105 mins by chemi-luminescence assayMore data for this Ligand-Target Pair
Affinity DataEC50: 19.9nMAssay Description:Agonist activity at human S1P1 receptor expressed in CHO-K1 EDG1 cells expressing beta-arrestin 2 after 105 mins by chemi-luminescence assayMore data for this Ligand-Target Pair
Affinity DataEC50: 19.9nMAssay Description:Agonist activity at human S1P1 receptor expressed in CHO-K1 EDG1 cells expressing beta-arrestin 2 after 105 mins by chemi-luminescence assayMore data for this Ligand-Target Pair
Affinity DataEC50: 7.94nMAssay Description:Agonist activity at human S1P1 receptor expressed in CHO-K1 EDG1 cells expressing beta-arrestin 2 after 105 mins by chemi-luminescence assayMore data for this Ligand-Target Pair
Affinity DataEC50: 5.62nMAssay Description:Agonist activity at human S1P1 receptor expressed in CHO-K1 EDG1 cells expressing beta-arrestin 2 after 105 mins by chemi-luminescence assayMore data for this Ligand-Target Pair
Affinity DataEC50: 5.01nMAssay Description:Agonist activity at human S1P3 receptor expressed in RBL cells assessed as [35S]GTPgammaS binding after 3 hrs by radiometric assayMore data for this Ligand-Target Pair
Affinity DataEC50: 3.16nMAssay Description:Agonist activity at human S1P1 receptor expressed in CHO-K1 EDG1 cells expressing beta-arrestin 2 after 105 mins by chemi-luminescence assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.26nMAssay Description:Agonist activity at human S1P1 receptor expressed in CHO-K1 EDG1 cells expressing beta-arrestin 2 after 105 mins by chemi-luminescence assayMore data for this Ligand-Target Pair
Affinity DataEC50: >3.16E+4nMAssay Description:Agonist activity at human S1P3 receptor expressed in RBL cells assessed as [35S]GTPgammaS binding after 3 hrs by radiometric assayMore data for this Ligand-Target Pair
Affinity DataEC50: >3.16E+4nMAssay Description:Agonist activity at human S1P3 receptor expressed in RBL cells assessed as [35S]GTPgammaS binding after 3 hrs by radiometric assayMore data for this Ligand-Target Pair
Affinity DataEC50: 19.9nMAssay Description:Agonist activity at human S1P1 receptor expressed in CHO-K1 EDG1 cells expressing beta-arrestin 2 after 105 mins by chemi-luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.16E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 6.76E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 8.40E+3nMAssay Description:Inhibition of CYP3A4 using vivid green as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 1.29E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 1.53E+4nMAssay Description:Inhibition of CYP3A4 using vivid red as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.78E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
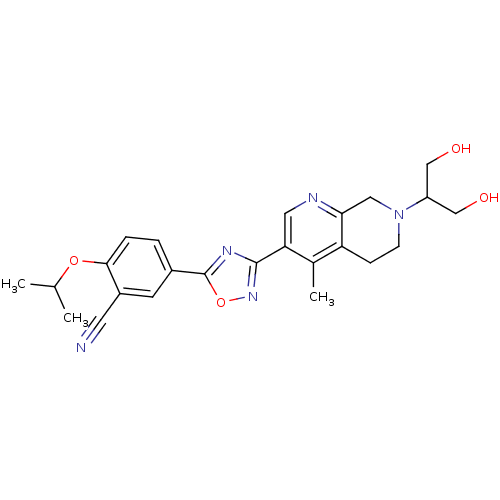
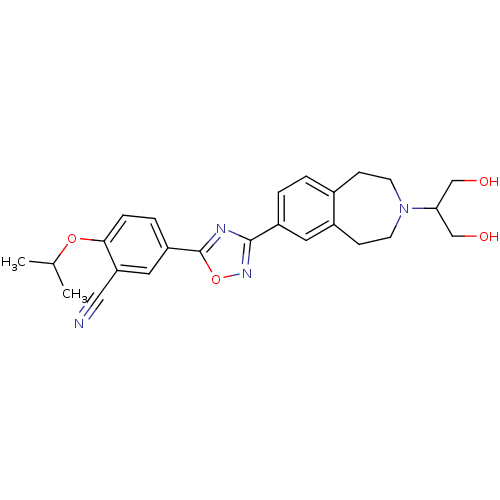
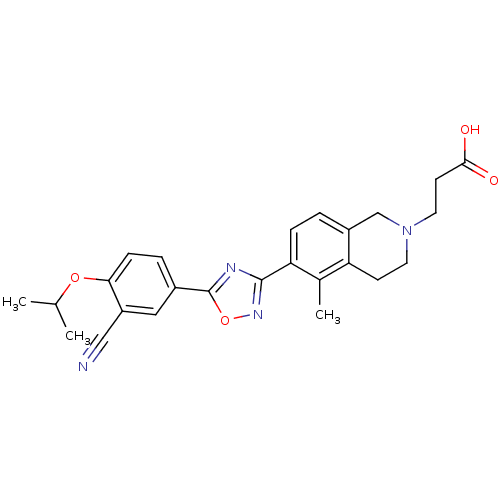
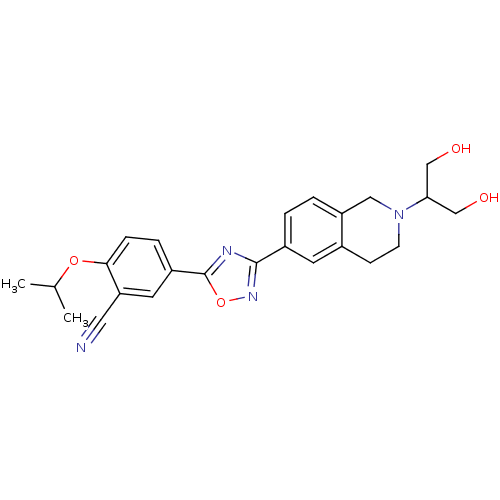
 3D Structure (crystal)
3D Structure (crystal)