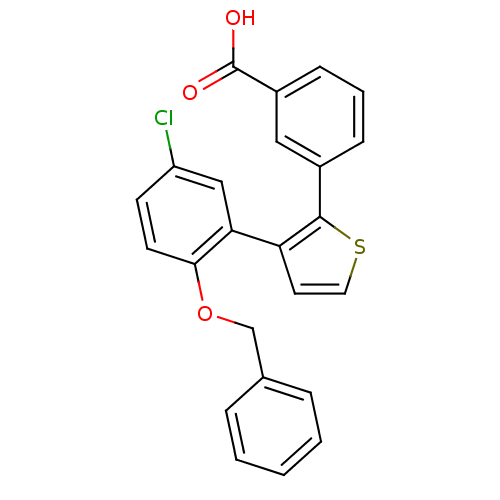
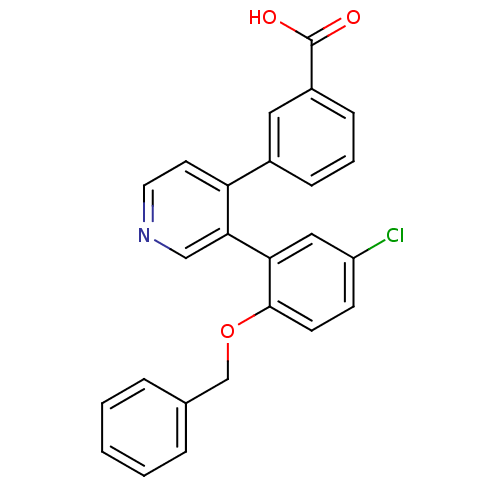
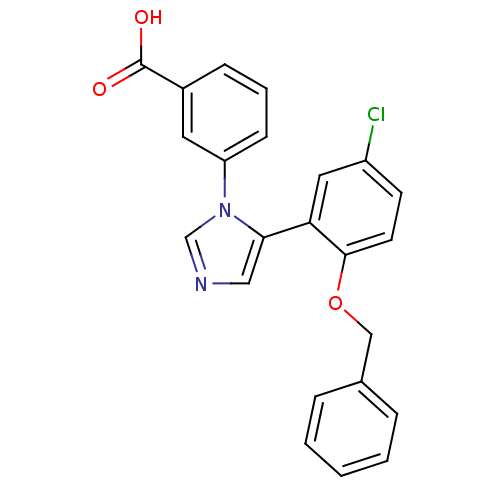
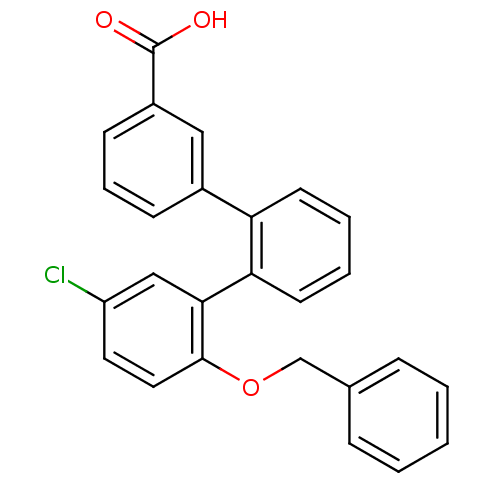
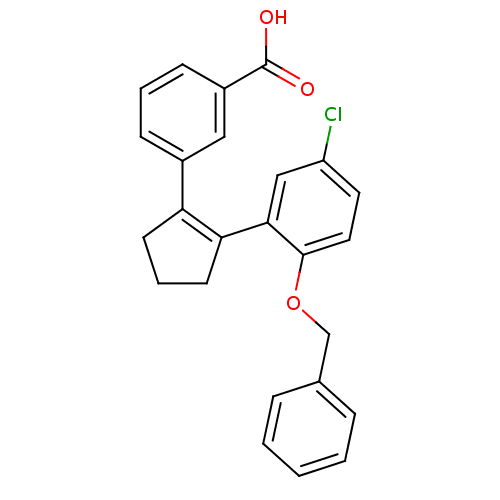
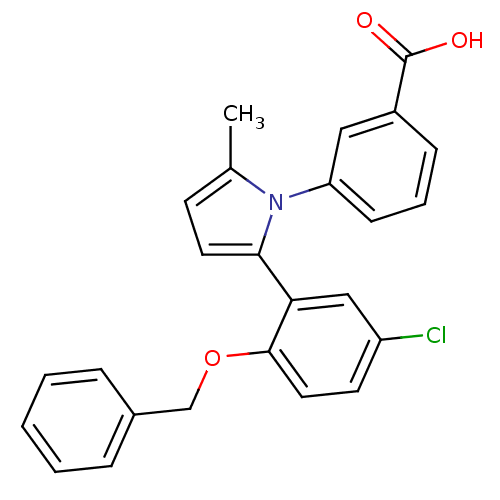
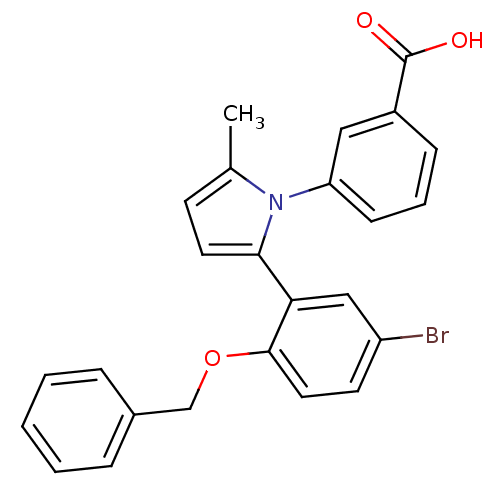
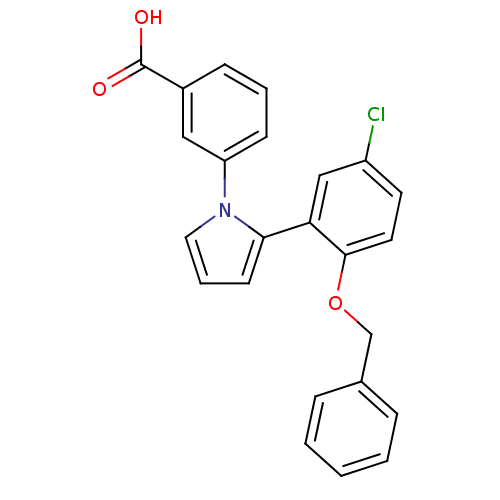
Report error Found 15 Enz. Inhib. hit(s) with all data for entry = 50017515
Affinity DataIC50: 5nMAssay Description:Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of CYP450 3A4More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of CYP450 2C9More data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of CYP450 2C9More data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibition of CYP450 2C9More data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+3nMAssay Description:Inhibition of CYP450 2C9More data for this Ligand-Target Pair
Affinity DataIC50: 6.50E+3nMAssay Description:Inhibition of CYP450 2C9More data for this Ligand-Target Pair
Affinity DataIC50: 6.80E+3nMAssay Description:Inhibition of CYP450 2C9More data for this Ligand-Target Pair
Affinity DataIC50: 7.30E+3nMAssay Description:Inhibition of CYP450 2C9More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP450 2C19More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP450 2D6More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP450 2C9More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP450 1A2More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP450 3A4More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Antagonist activity against PGE2 activated EP1 receptor assessed as ability to inhibit intracellular calcium mobilisation by FLIPRMore data for this Ligand-Target Pair