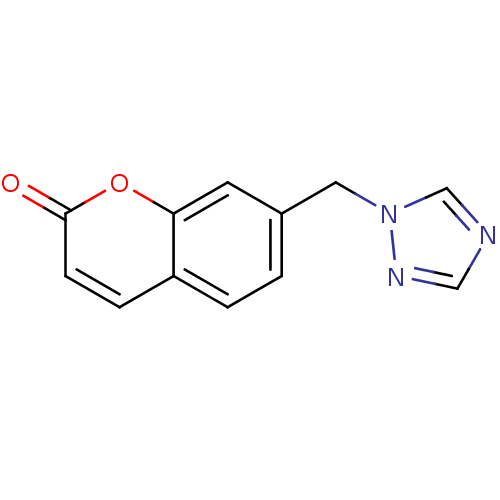
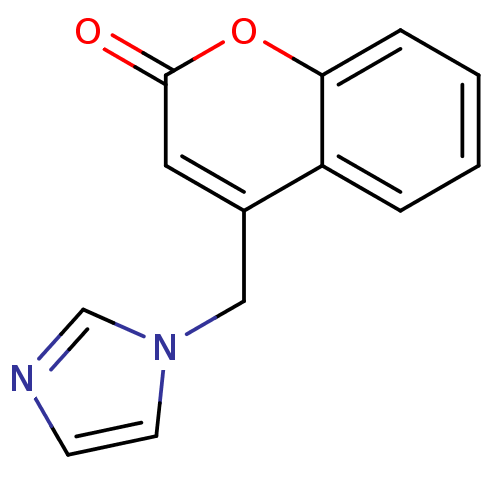
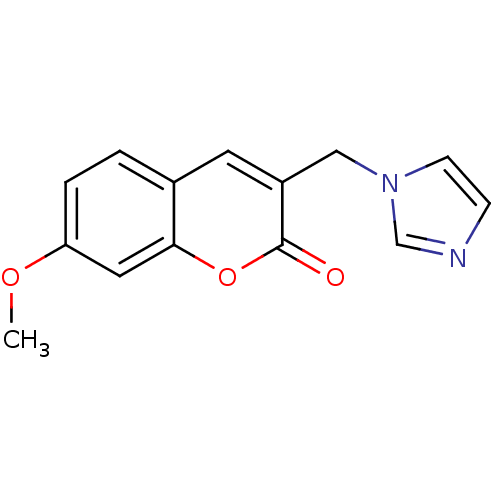
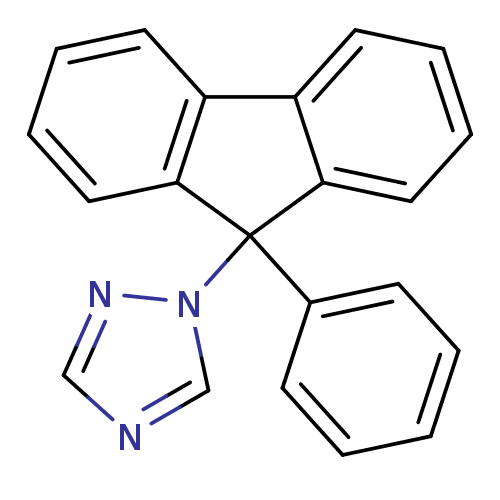
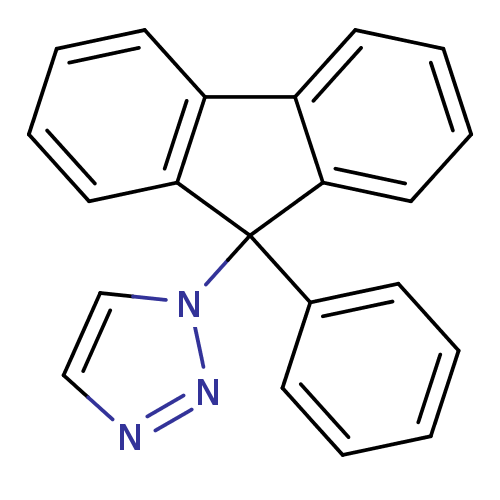
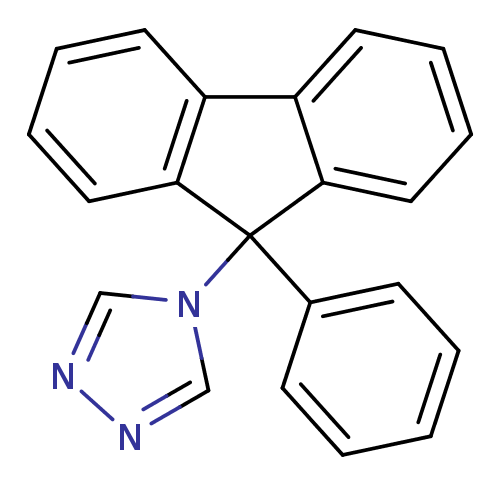
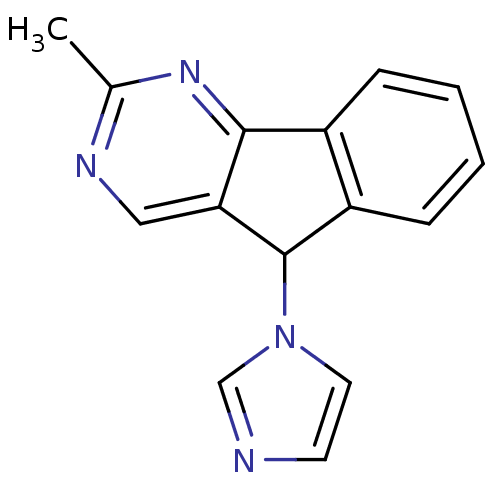
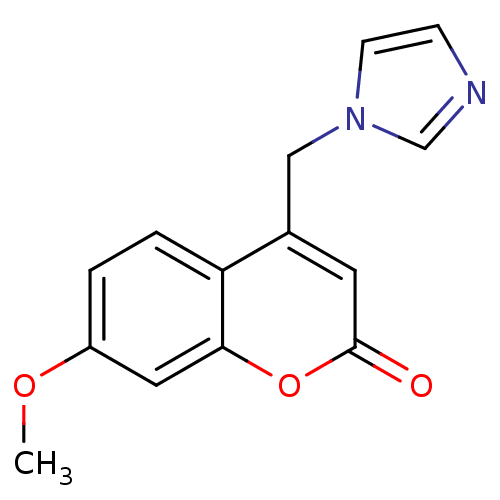
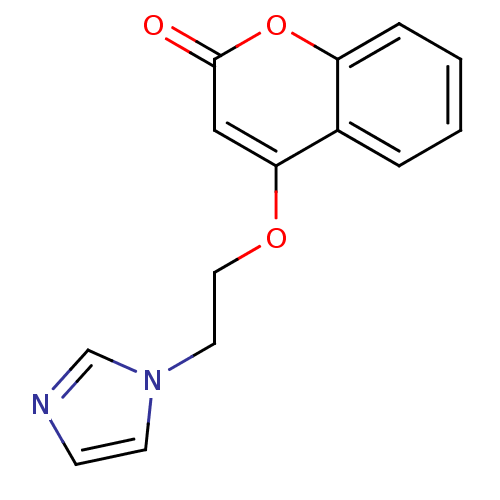
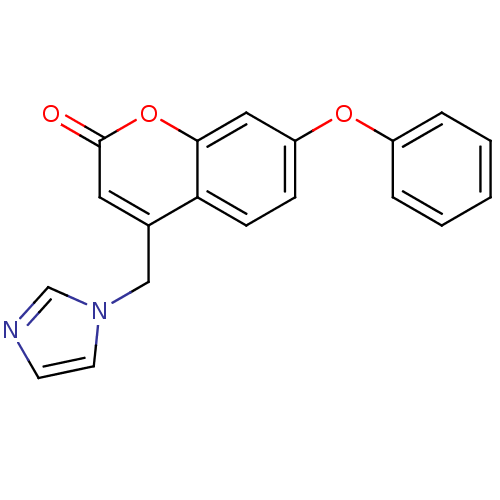
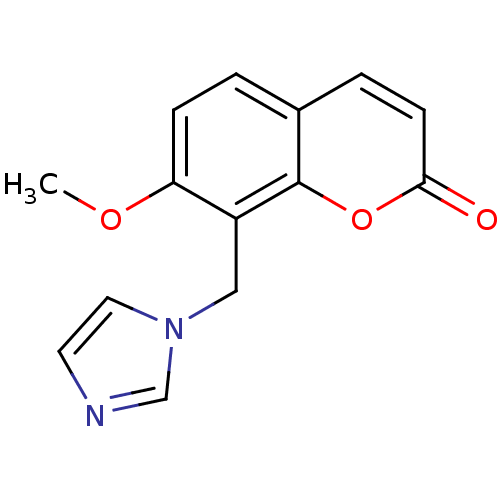
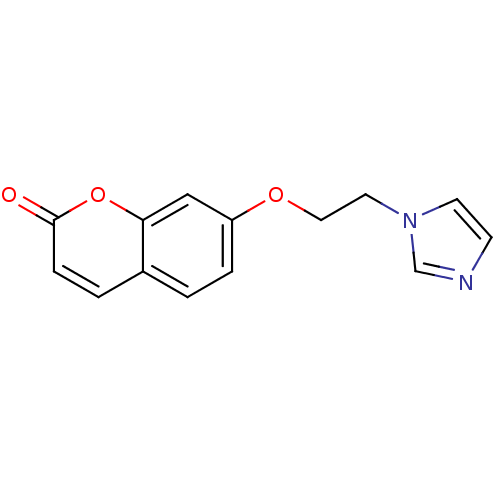
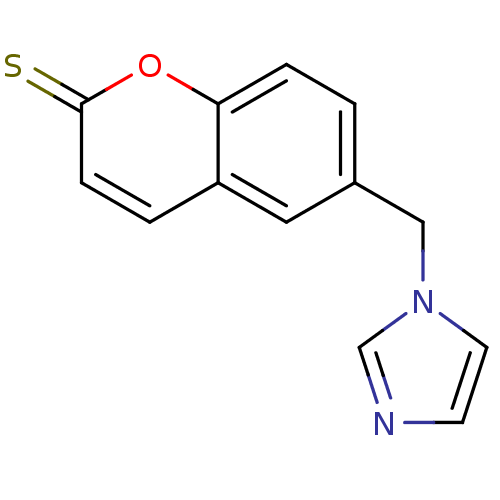
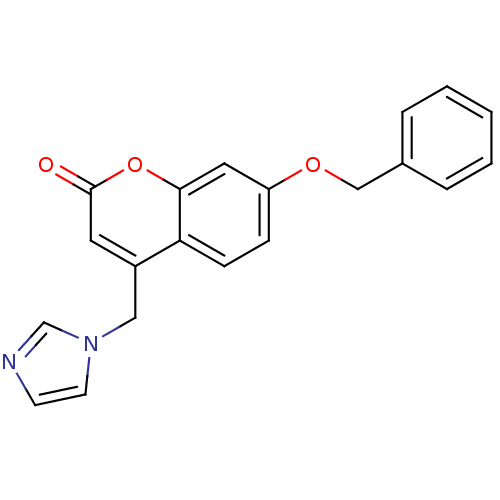
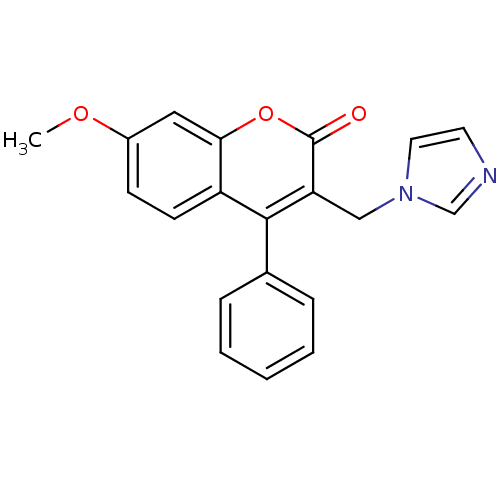
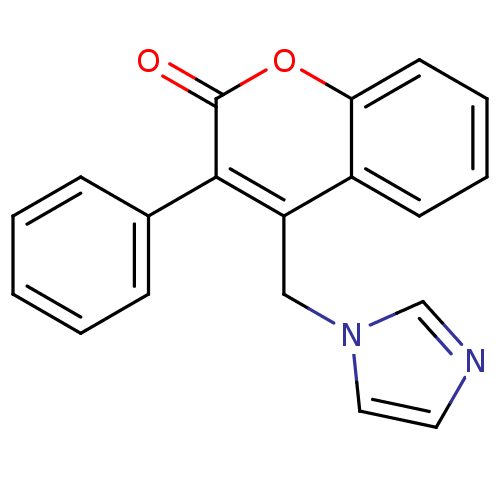
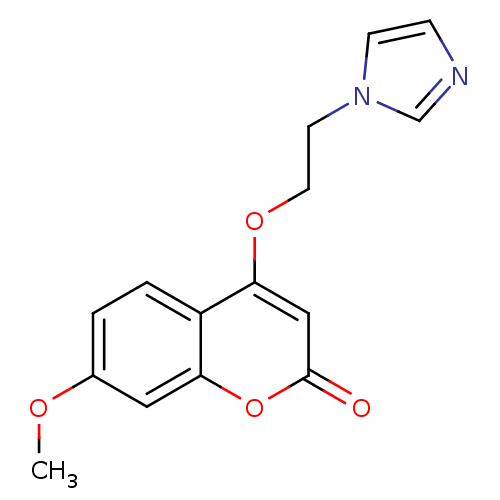
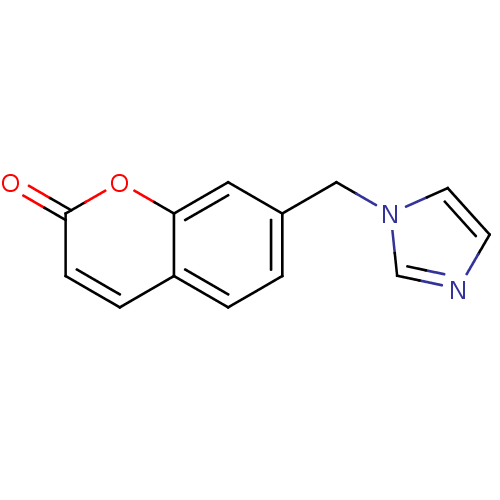
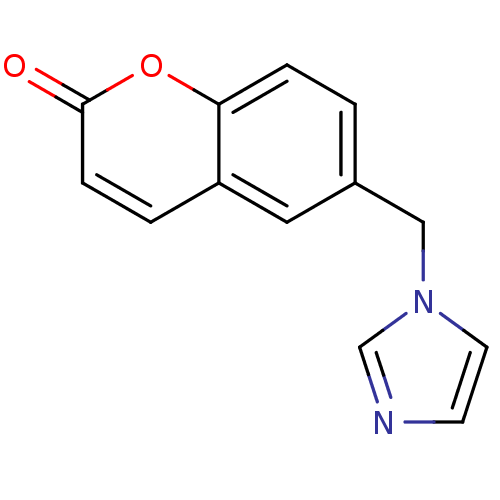
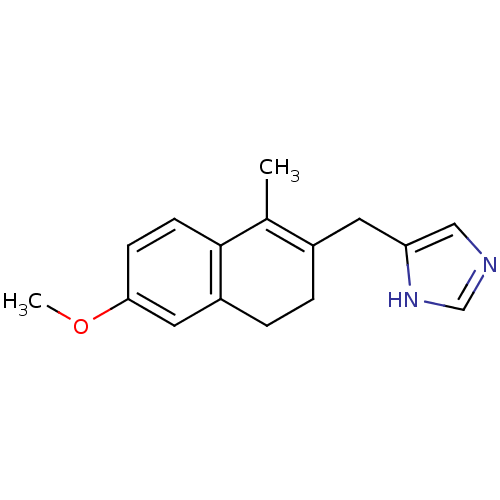
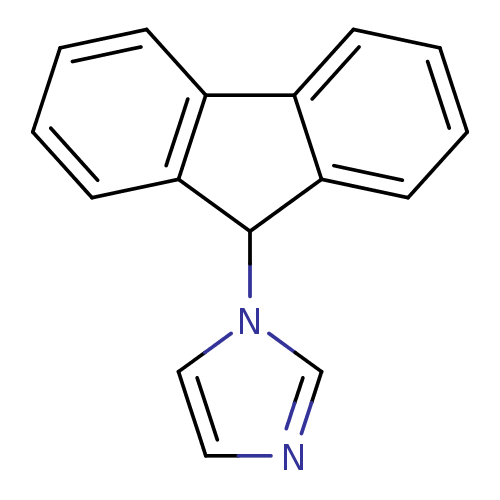
Report error Found 50 Enz. Inhib. hit(s) with all data for entry = 1161
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 51nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 74nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 106nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 144nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 150nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 168nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 280nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 680nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 760nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 1.13E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 2.82E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 2.85E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 2.85E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.13E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DataIC50: 2.66E+4nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair