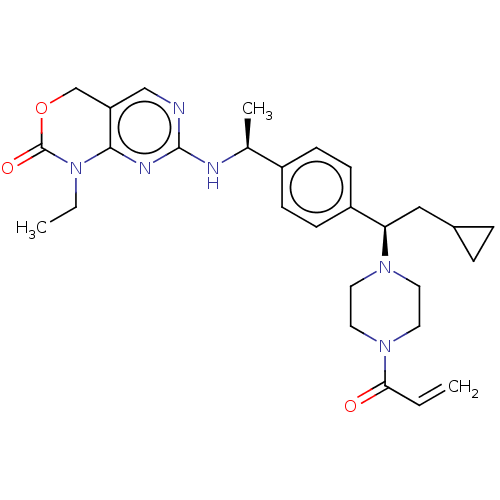
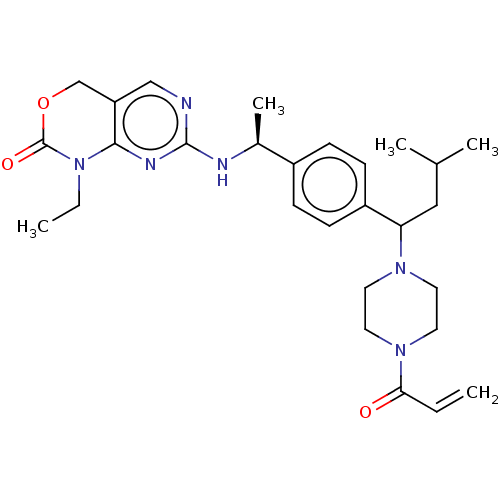
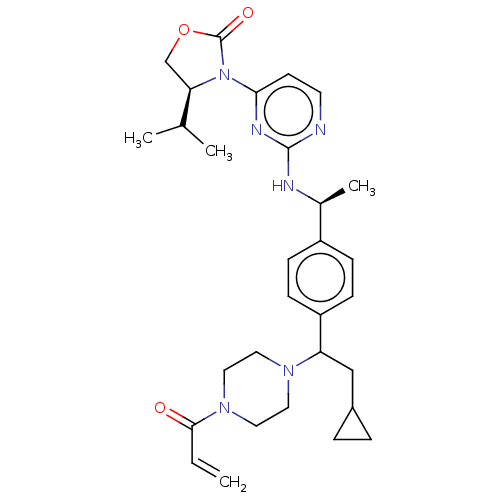
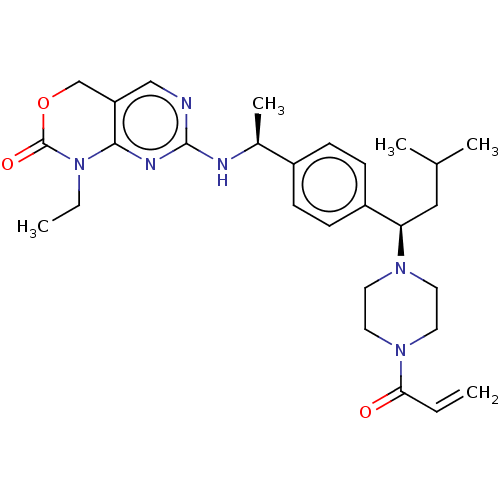
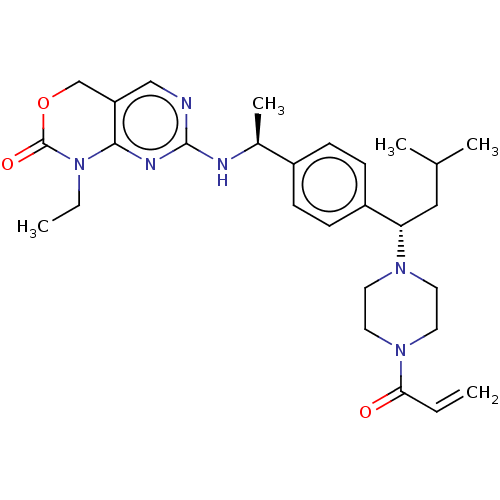
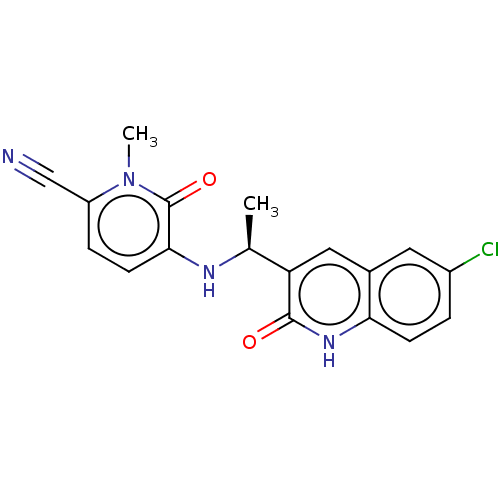
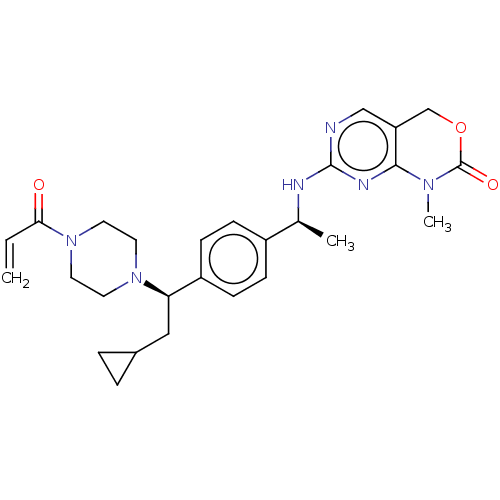
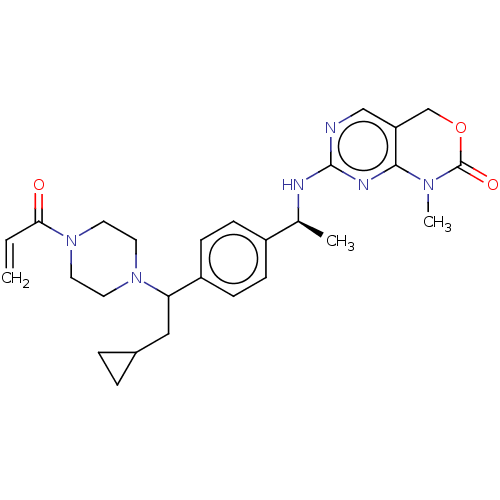
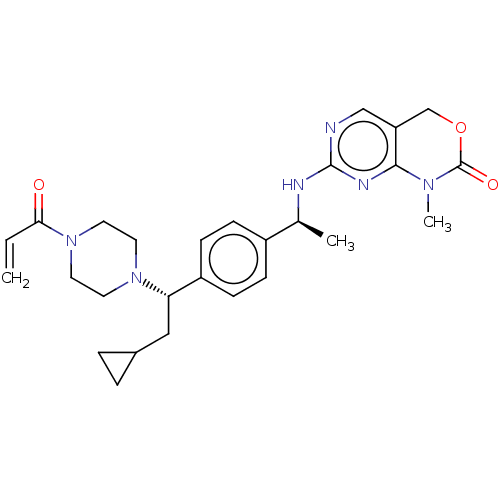
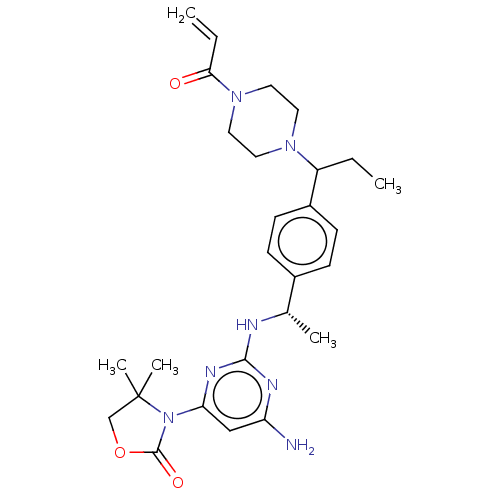
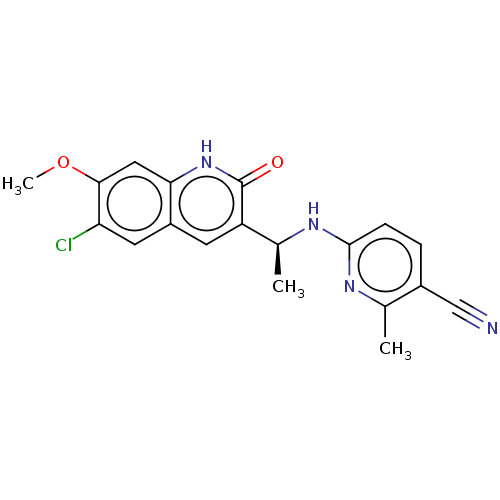
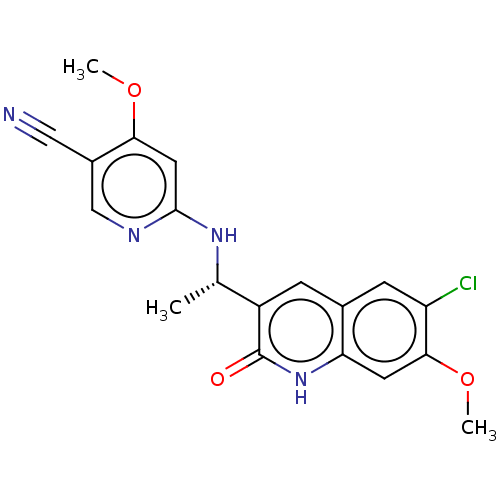
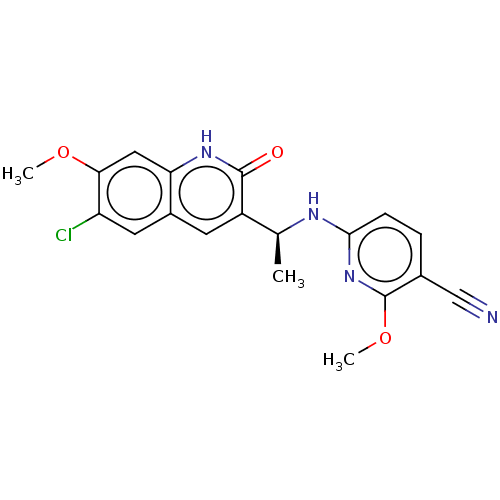
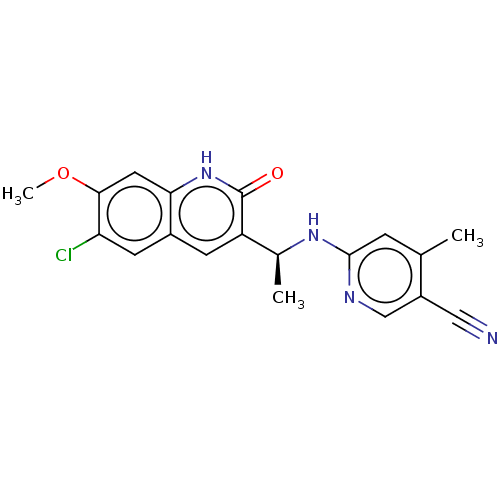
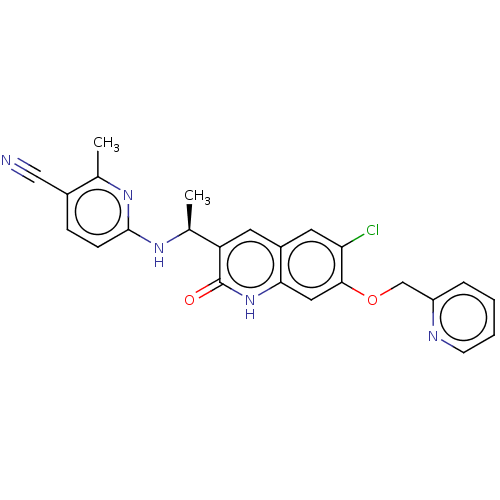
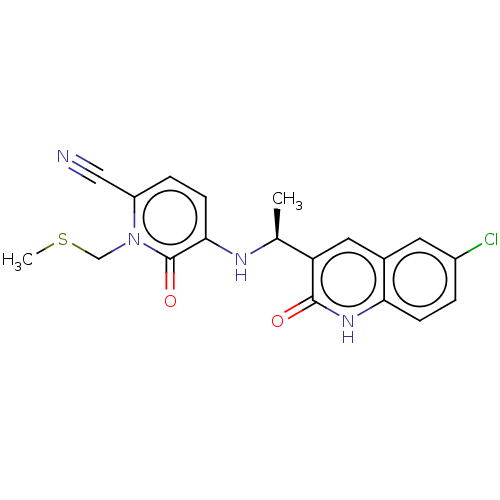
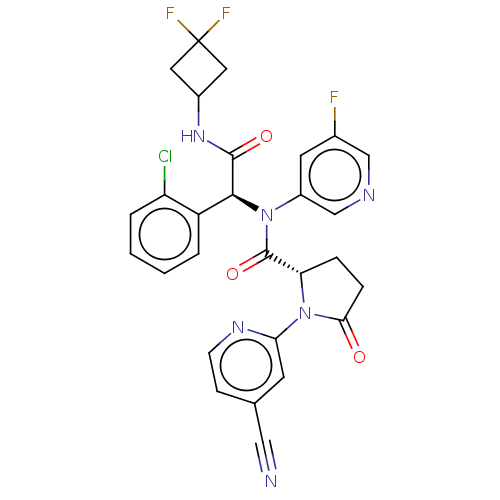
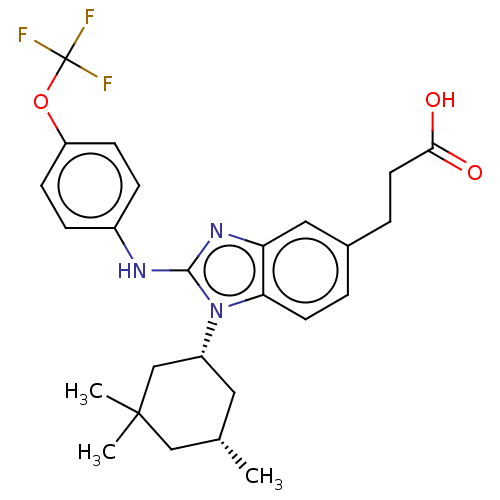
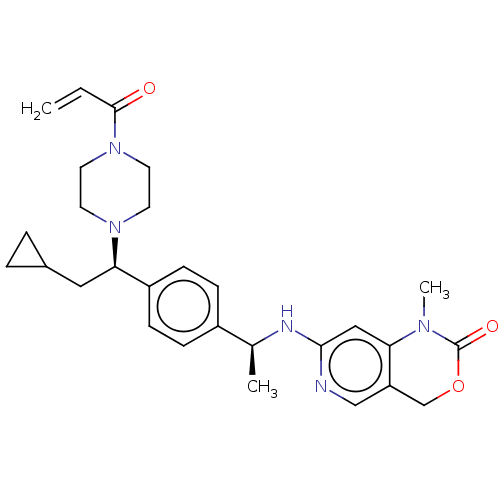
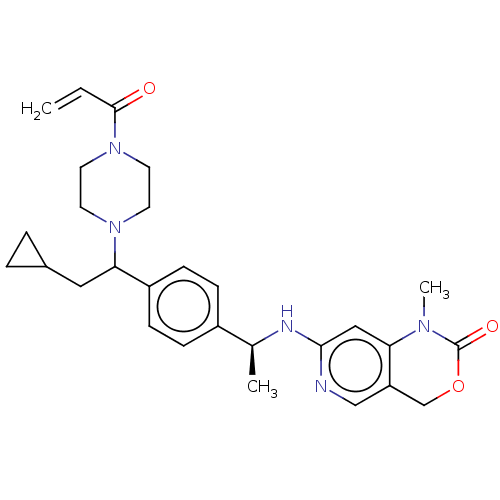
Report error Found 2342 Enz. Inhib. hit(s) with Target = 'Isocitrate dehydrogenase [NADP] cytoplasmic [R132C]'
Affinity DataIC50: 0nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 0nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 2.49nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 2.49nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 2.49nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 3.71nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 3.71nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 3.71nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMT: 2°CAssay Description:RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:Compound 1 was not initially reported as having the greatest biochemical potency compared to reports for certain other small molecule inhibitors of m...More data for this Ligand-Target Pair
Affinity DataIC50: 4.31nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 4.31nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 4.31nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 5.05nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 5.05nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 5.05nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 5.08nMAssay Description:IDH1 mutant (R132H and R132C) and IDH2 mutant (R140Q and R172K) proteins containing N-terminal His-tag are expressed in E. coli and purified using ni...More data for this Ligand-Target Pair
Affinity DataIC50: 5.08nMAssay Description:IDH1 mutant (R132H and R132C) and IDH2 mutant (R140Q and R172K) proteins containing N-terminal His-tag are expressed in E. coli and purified using ni...More data for this Ligand-Target Pair
Affinity DataIC50: 5.08nMAssay Description:IDH1 mutant (R132H and R132C) and IDH2 mutant (R140Q and R172K) proteins containing N-terminal His-tag are expressed in E. coli and purified using ni...More data for this Ligand-Target Pair
Affinity DataIC50: 5.08nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 5.08nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 5.08nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 5.08nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 5.08nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 5.08nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Compound 1 was not initially reported as having the greatest biochemical potency compared to reports for certain other small molecule inhibitors of m...More data for this Ligand-Target Pair
Affinity DataIC50: 8.69nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 8.69nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 8.69nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 8.89nMAssay Description:IDH1 mutant (R132H and R132C) and IDH2 mutant (R140Q and R172K) proteins containing N-terminal His-tag are expressed in E. coli and purified using ni...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Assays were performed in a 384-well black plate. An aliquot of 250 nL of compound was incubated with 10 μL of 30 nM IDH1-R132H or 10 nM IDH1-R13...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Assays were performed in a 384-well black plate. An aliquot of 250 nL of compound was incubated with 10 μL of 30 nM IDH1-R132H or 10 nM IDH1-R13...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Assays were performed in a 384-well black plate. An aliquot of 250 nL of compound was incubated with 10 μL of 30 nM IDH1-R132H or 10 nM IDH1-R13...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Assays were performed in a 384-well black plate. An aliquot of 250 nL of compound was incubated with 10 μL of 30 nM IDH1-R132H or 10 nM IDH1-R13...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Assays were performed in a 384-well black plate. An aliquot of 250 nL of compound was incubated with 10 μL of 30 nM IDH1-R132H or 10 nM IDH1-R13...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:HCT116 isogenic IDH1-R132H and IDH1-R132C mutant cells were cultured in growth media (McCoy's 5 A, 10% fetal bovine serum, 1× antibiotic-antimyco...More data for this Ligand-Target Pair
Affinity DataIC50: 12.9nMAssay Description:Assays were performed in a 384-well black plate. An aliquot of 250 nL of compound was incubated with 10 μL of 30 nM IDH1-R132H or 10 nM IDH1-R13...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Compound 1 was not initially reported as having the greatest biochemical potency compared to reports for certain other small molecule inhibitors of m...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Compound 1 was not initially reported as having the greatest biochemical potency compared to reports for certain other small molecule inhibitors of m...More data for this Ligand-Target Pair
Affinity DataIC50: 15.1nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 15.1nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 15.1nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 15.6nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 15.6nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 15.6nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 15.6nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 15.6nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 15.6nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 16.5nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
Affinity DataIC50: 16.5nMAssay Description:IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas...More data for this Ligand-Target Pair
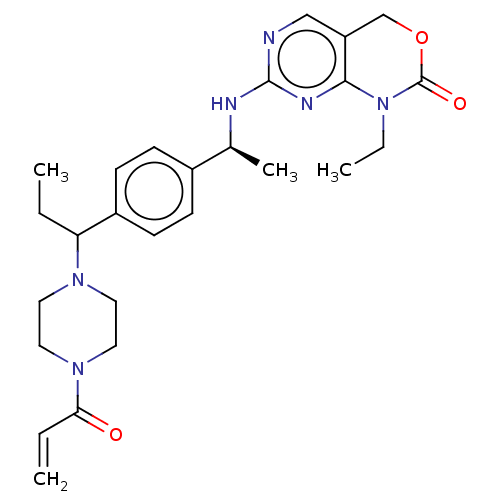
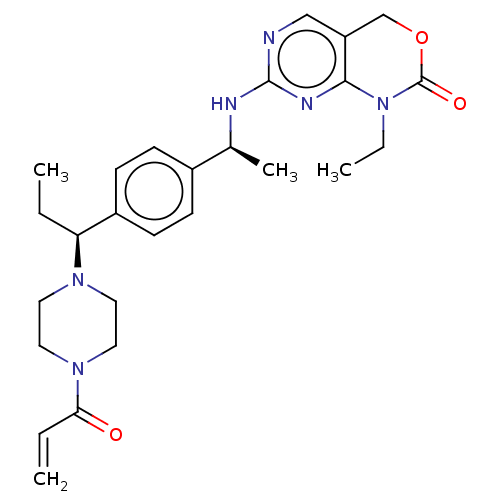

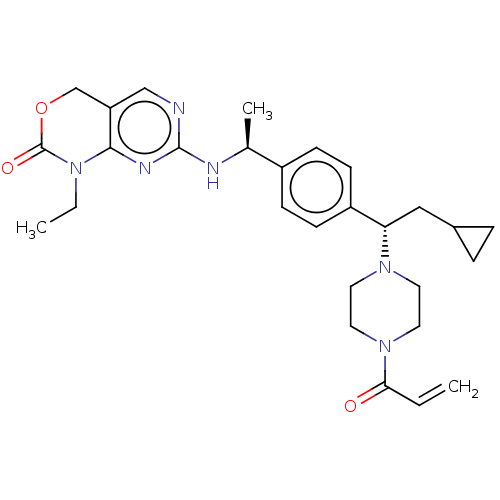
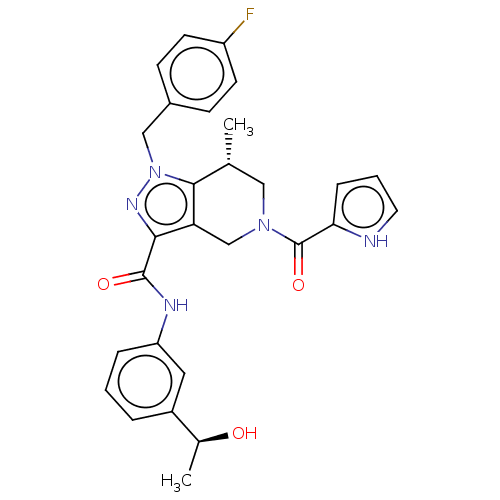
 3D Structure (crystal)
3D Structure (crystal)