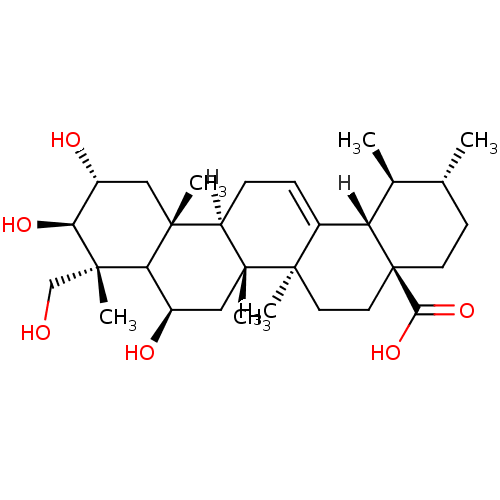
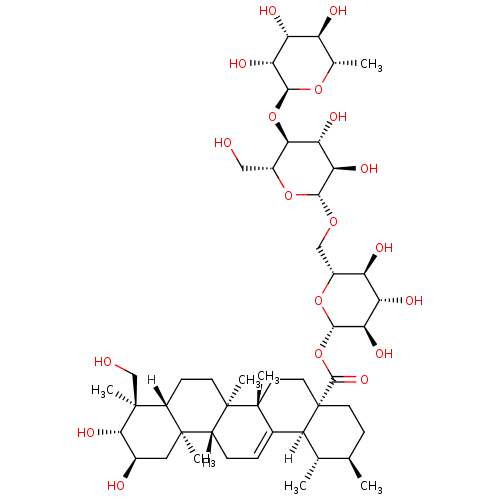
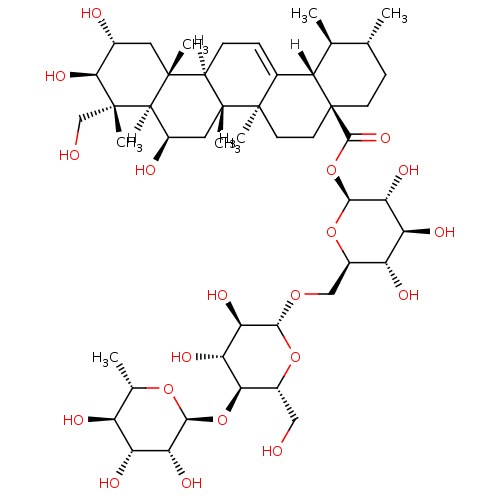
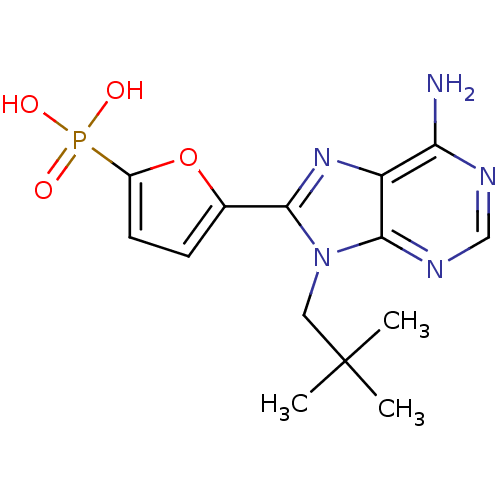
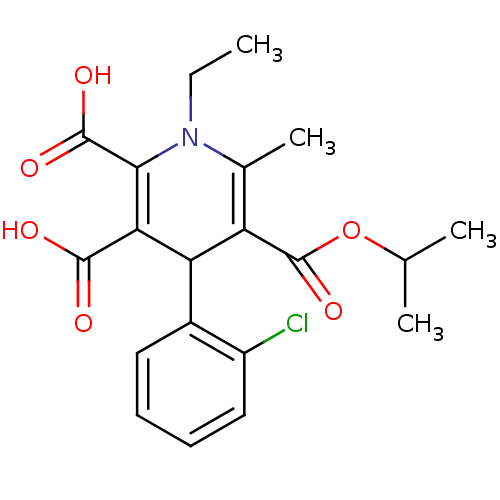
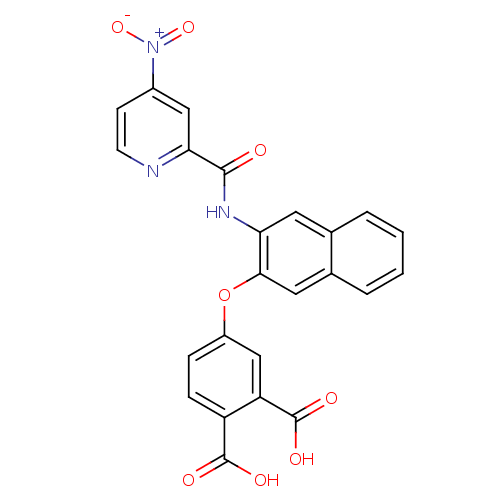
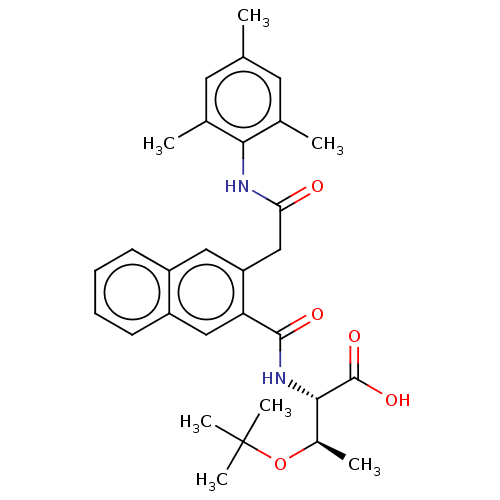
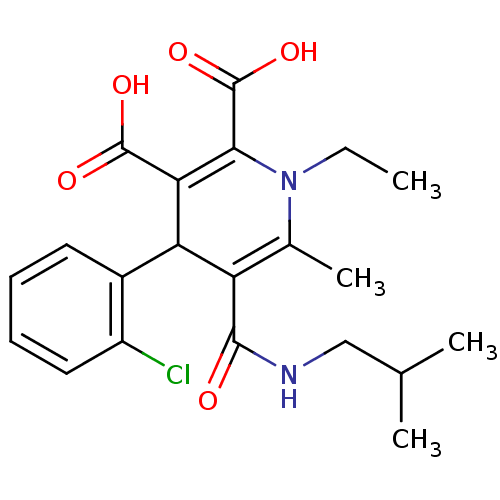
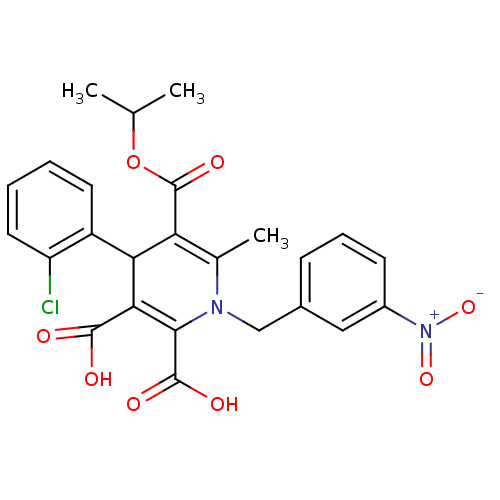
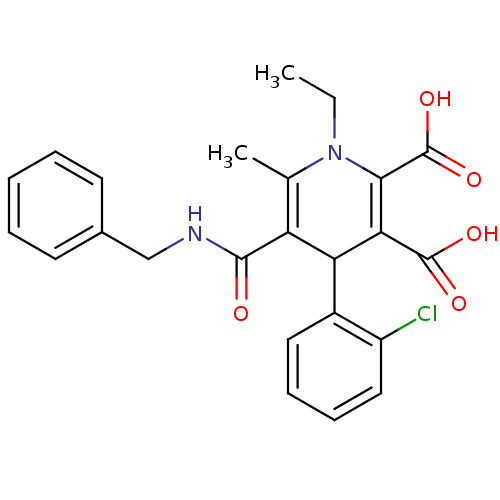
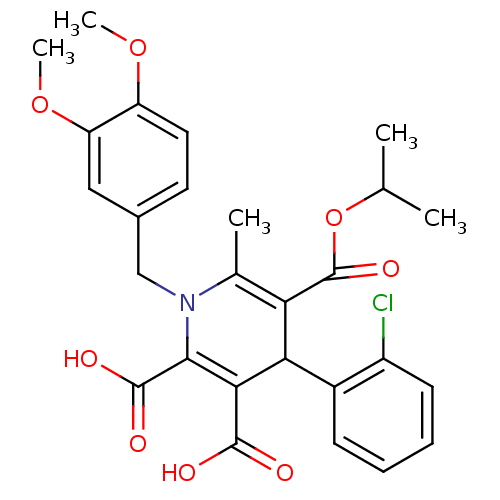
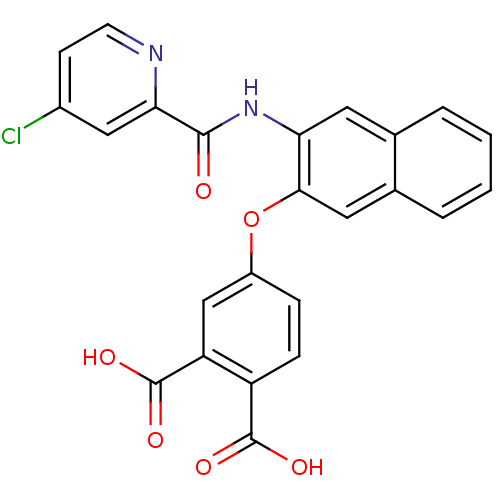
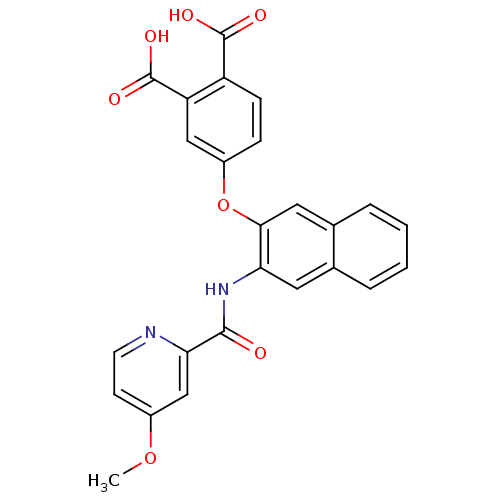
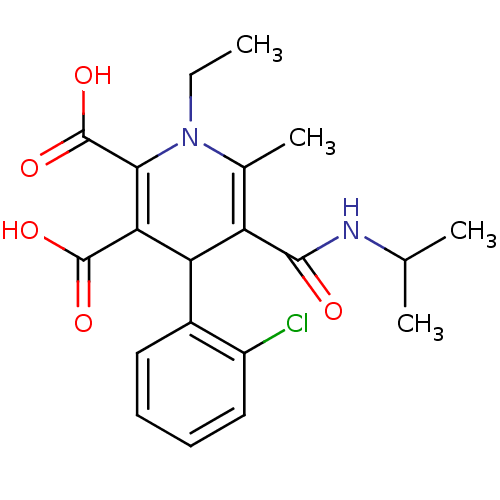
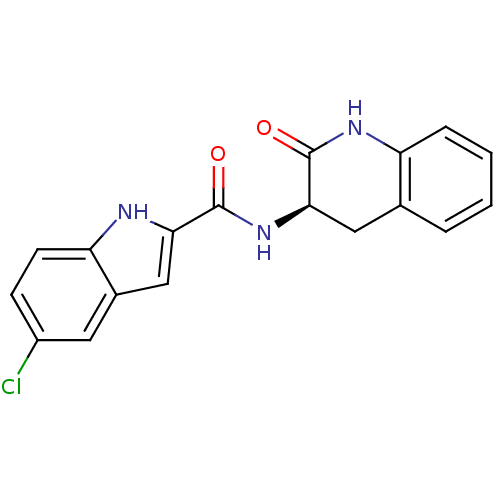
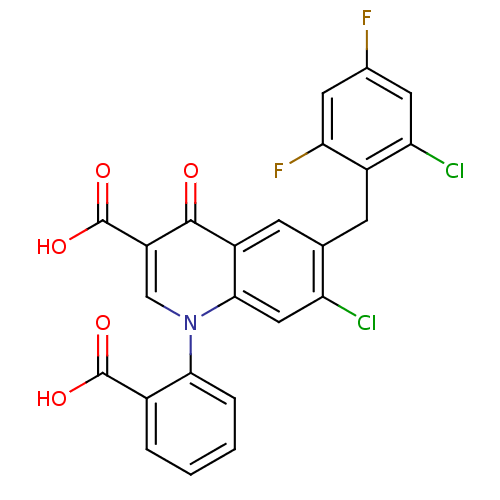
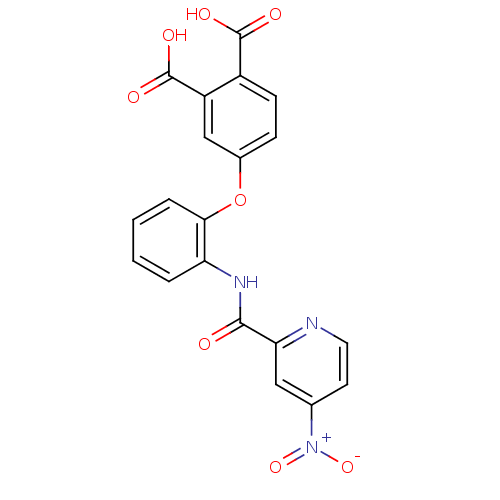
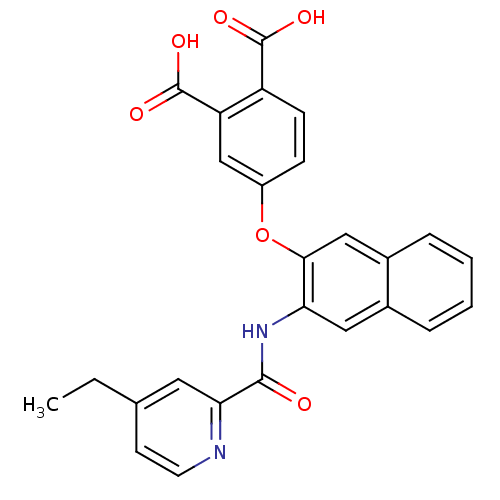
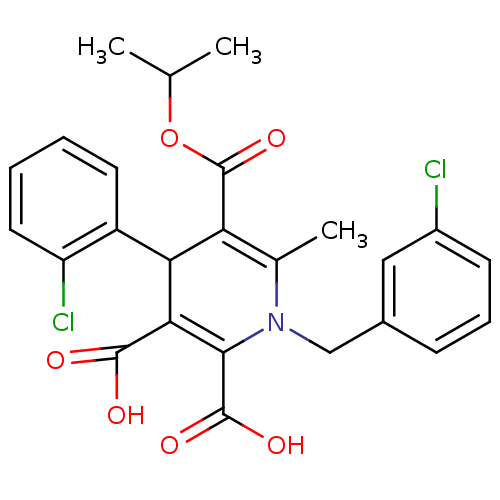
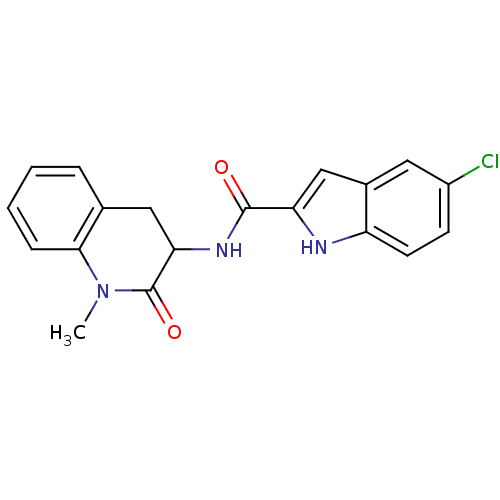
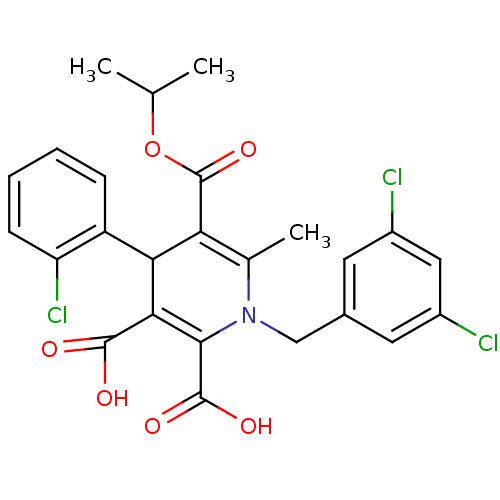
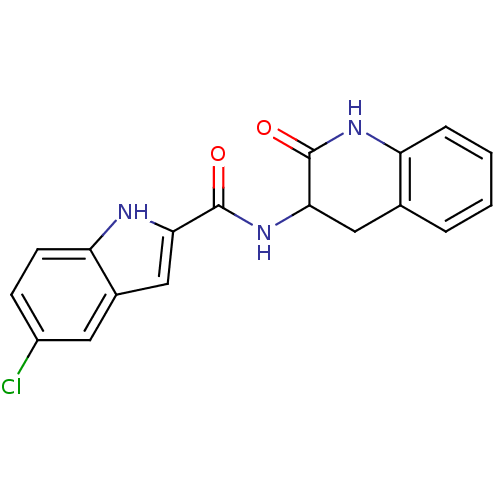
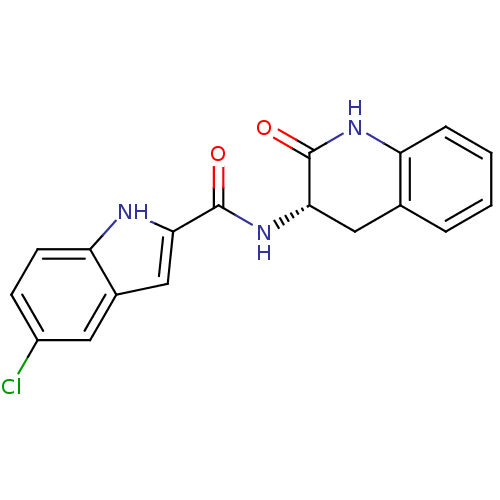
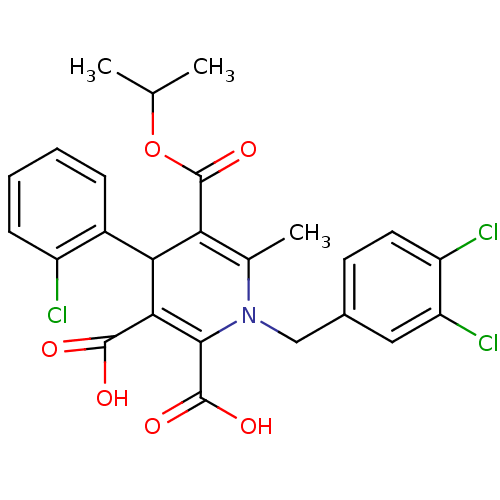
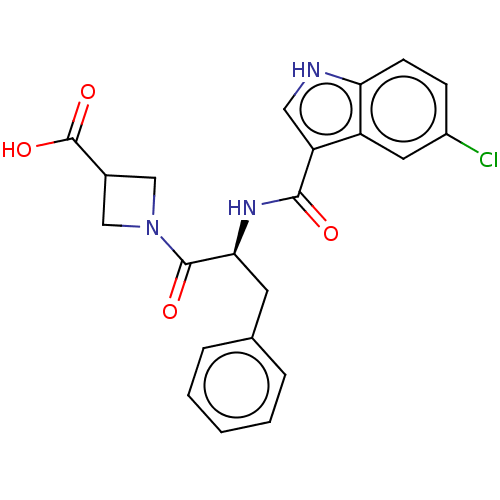
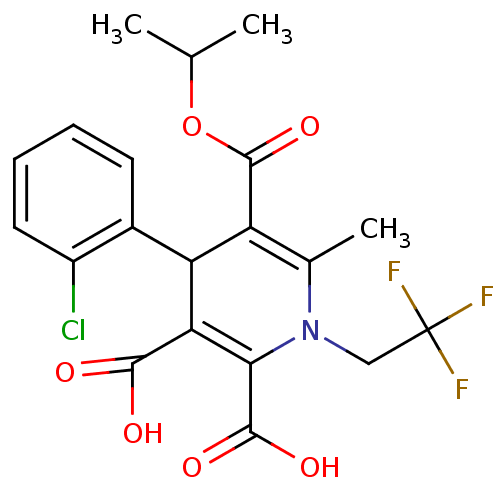
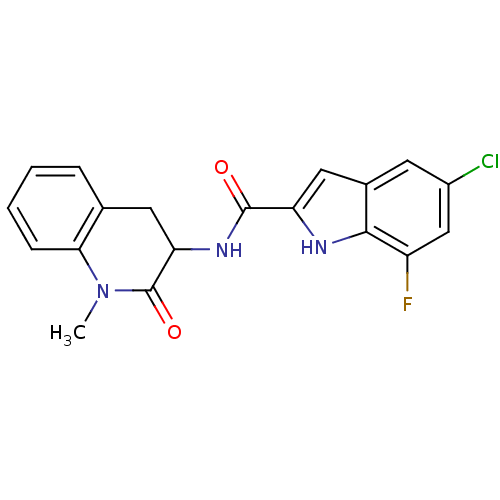
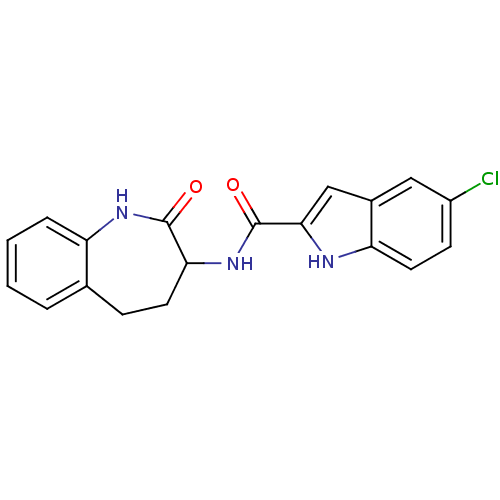
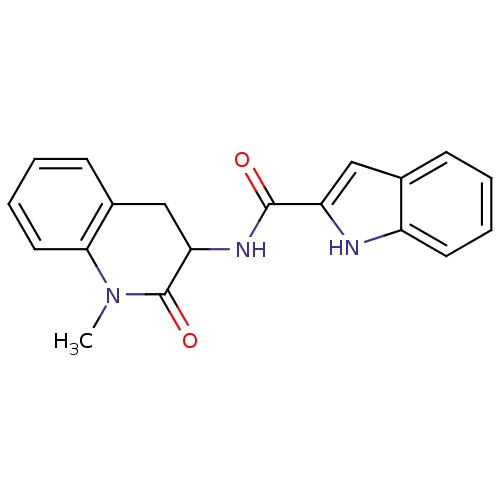
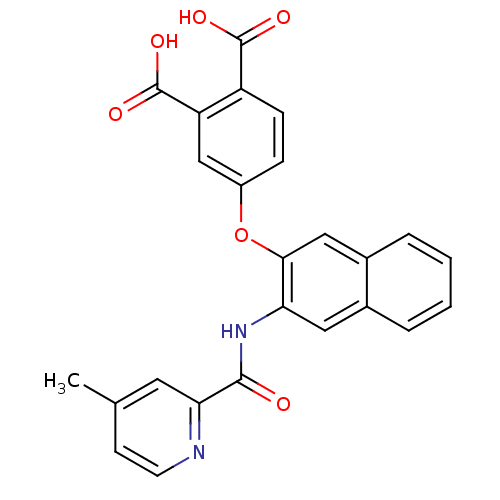
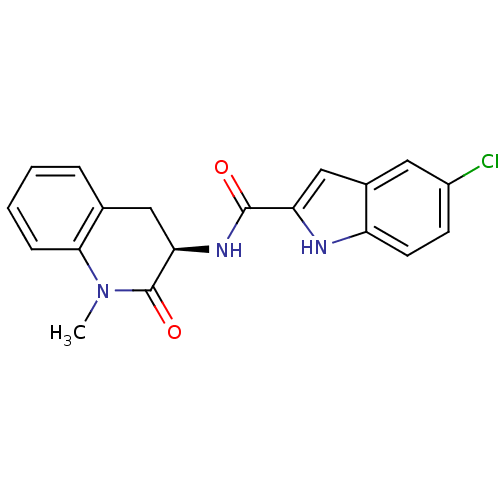
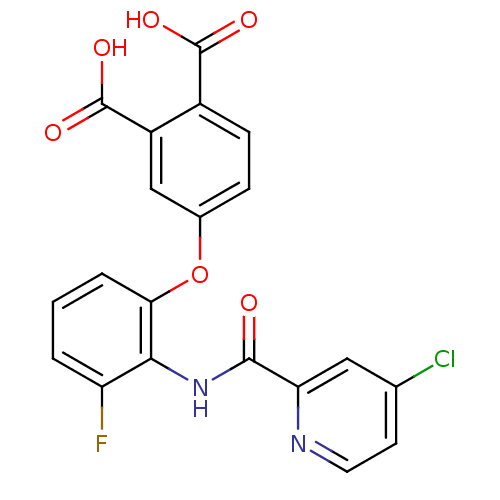
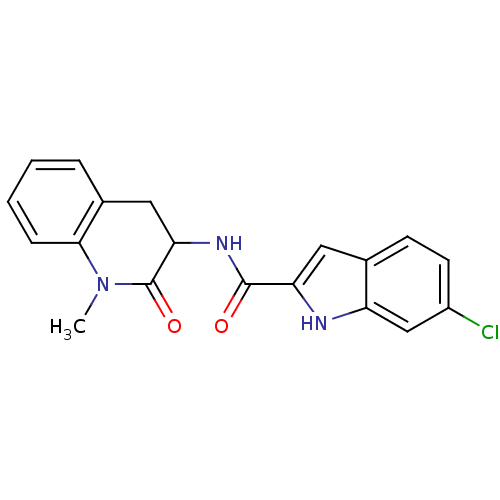
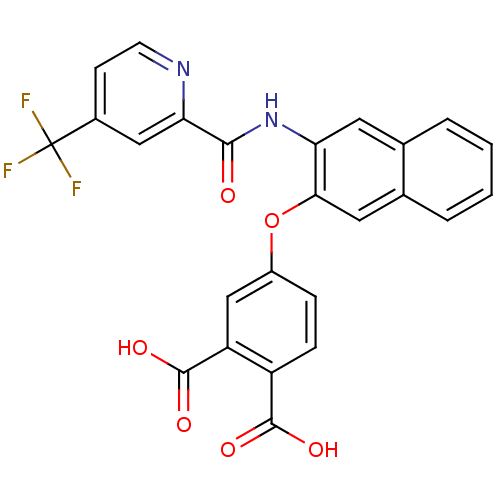
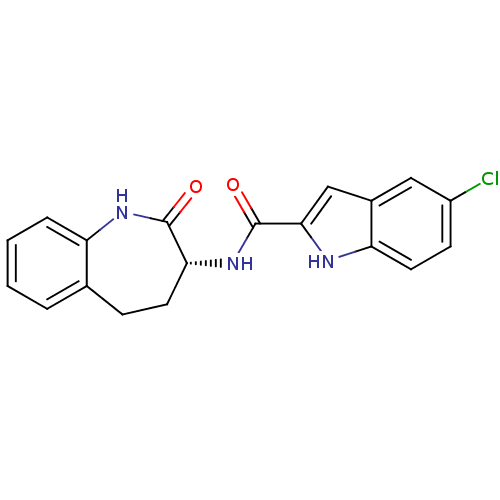
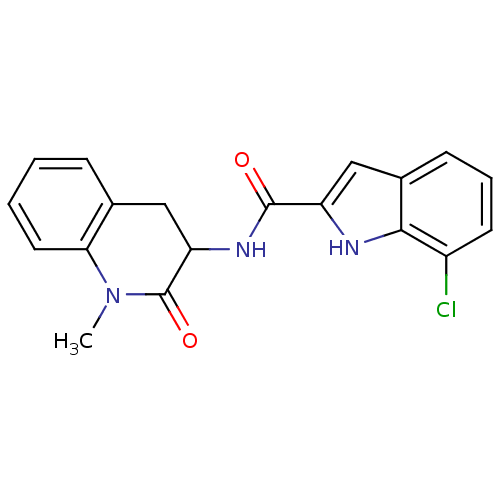
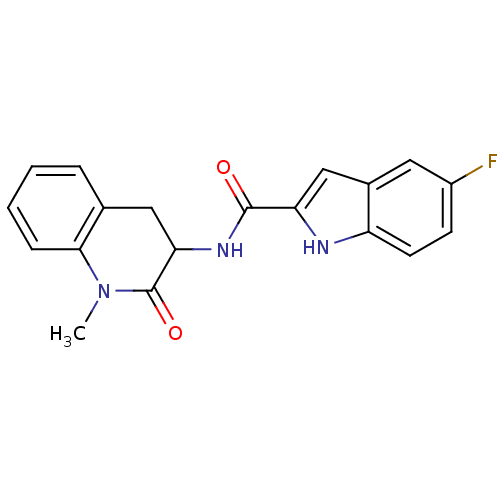
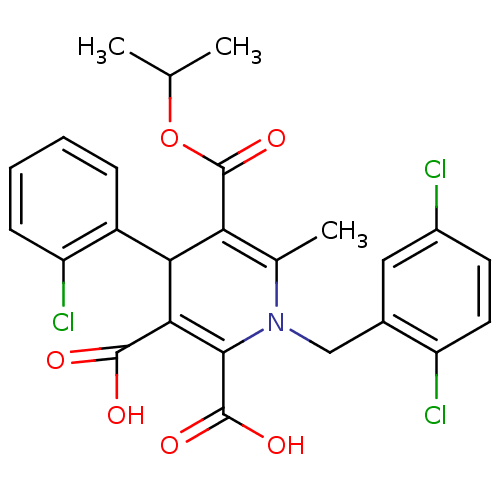
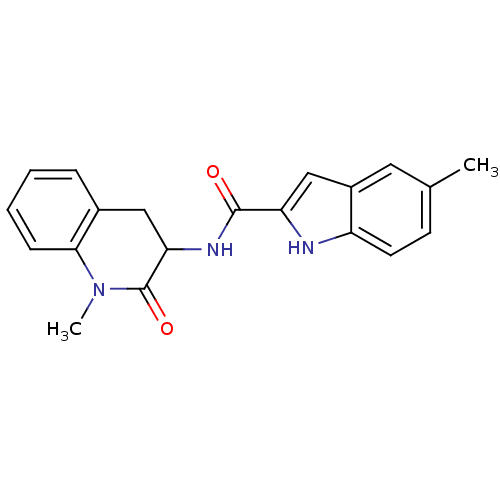
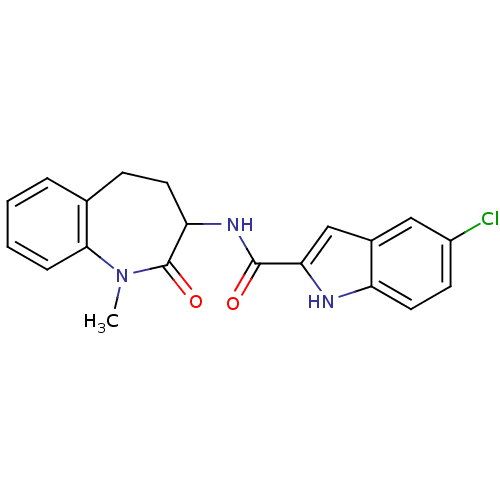
Report error Found 980 Enz. Inhib. hit(s) with Target = 'Glycogen phosphorylase, muscle form'
Affinity DatapH: 7.2 T: 2°CAssay Description:The activity of the compounds is determined by measuring the inhibitory effect of the compounds in the direction of glycogen synthesis, the conversio...More data for this Ligand-Target Pair
Affinity DatapH: 7.2 T: 2°CAssay Description:The activity of the compounds is determined by measuring the inhibitory effect of the compounds in the direction of glycogen synthesis, the conversio...More data for this Ligand-Target Pair
Affinity DatapH: 7.2 T: 2°CAssay Description:The activity of the compounds is determined by measuring the inhibitory effect of the compounds in the direction of glycogen synthesis, the conversio...More data for this Ligand-Target Pair
Affinity DatapH: 7.2 T: 2°CAssay Description:RMGPa (Rabbit Muscle Glycogen Phosphorylase a) activity was measured in the direction of glycogen synthesis by the formation of inorganic phosphate f...More data for this Ligand-Target Pair
Affinity DataEC50: >2.50E+5nMAssay Description:Inhibition of rabbit muscle glycogen phosphorylaseMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+5nMAssay Description:Inhibition of rabbit muscle glycogen phosphorylaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Binding affinity to rabbit muscular GPb by NMR binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibitory activity against HMGP(human muscle glycogen phosphorylase)More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of rabbit muscle glycogen phosphorylase a assessed as inhibition of release of phosphate from glucose-1-phosphate after 30 mins by spectro...More data for this Ligand-Target Pair
Affinity DataIC50: 8.28nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibitory activity against HMGP(human muscle glycogen phosphorylase)More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 10.6nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibitory activity against HMGP(human muscle glycogen phosphorylase)More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibitory activity against HMGP(human muscle glycogen phosphorylase)More data for this Ligand-Target Pair
Affinity DataIC50: 12.9nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 19.9nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Binding affinity to rabbit muscular GPb by NMR binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibitory activity against HMGP(human muscle glycogen phosphorylase)More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Inhibitory activity against HMGP(human muscle glycogen phosphorylase)More data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of rabbit muscle glycogen phosphorylase a assessed as inhibition of release of phosphate from glucose-1-phosphate after 30 mins by spectro...More data for this Ligand-Target Pair
Affinity DataIC50: 52nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:Inhibitory activity against HMGP(human muscle glycogen phosphorylase)More data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 58nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibitory activity against HMGP(human muscle glycogen phosphorylase)More data for this Ligand-Target Pair
Affinity DataIC50: 69nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 73nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibitory activity against HMGP(human muscle glycogen phosphorylase)More data for this Ligand-Target Pair
Affinity DataIC50: 83nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 88nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 89nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 91nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 98nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 105nMAssay Description:Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 109nMAssay Description:Inhibitory activity against HMGP(human muscle glycogen phosphorylase)More data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibitory activity against HMGP(human muscle glycogen phosphorylase)More data for this Ligand-Target Pair