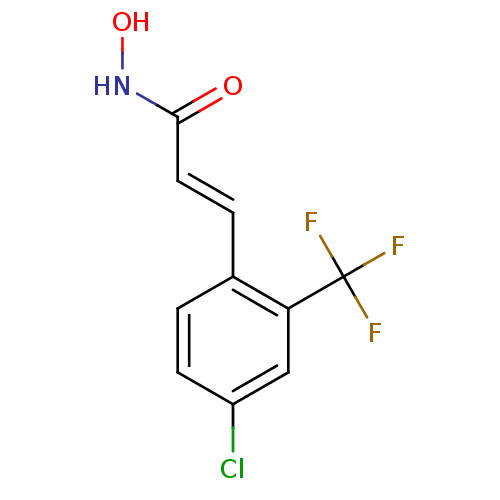
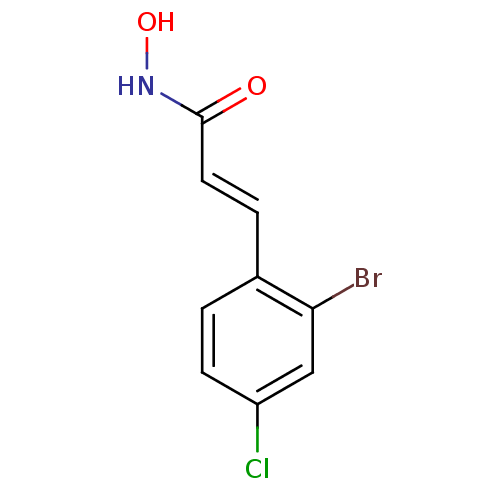
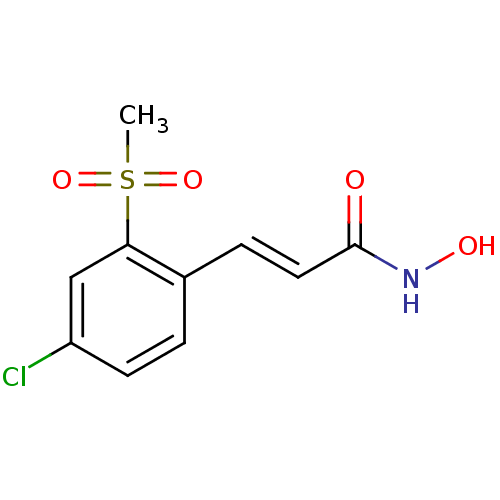
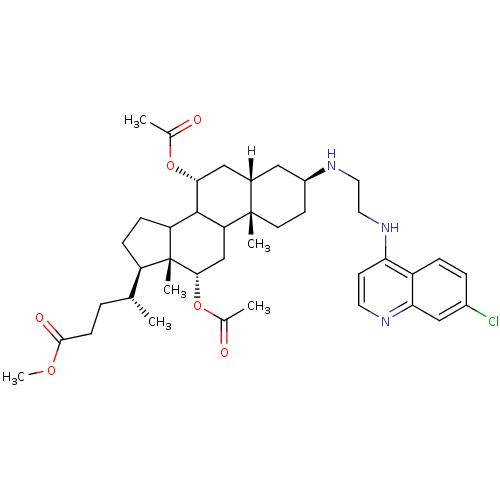
Report error Found 133 Enz. Inhib. hit(s) with Target = 'Botulinum neurotoxin type A2 [1-425]'
Affinity DatapH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont...More data for this Ligand-Target Pair
Affinity DataIC50: 410nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 700nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 800nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 900nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMpH: 7.4 T: 2°CAssay Description:Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMpH: 7.4 T: 2°CAssay Description:Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+3nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+3nMpH: 7.4 T: 2°CAssay Description:Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont...More data for this Ligand-Target Pair
Affinity DataIC50: 5.10E+3nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 5.45E+3nMAssay Description:The fluorescence peptide cleavage assay was performed in a 96-well plate in which each reaction mixture contained MAPKKide, LF (List Biological Labor...More data for this Ligand-Target Pair
Affinity DataIC50: 5.60E+3nMpH: 7.4 T: 2°CAssay Description:Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont...More data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+3nMpH: 7.4 T: 2°CAssay Description:Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont...More data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: 7.20E+3nMpH: 7.4 T: 2°CAssay Description:Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont...More data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+3nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 8.18E+3nMAssay Description:The fluorescence peptide cleavage assay was performed in a 96-well plate in which each reaction mixture contained MAPKKide, LF (List Biological Labor...More data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+3nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 9.72E+3nMAssay Description:The fluorescence peptide cleavage assay was performed in a 96-well plate in which each reaction mixture contained MAPKKide, LF (List Biological Labor...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 7.4 T: 2°CAssay Description:Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont...More data for this Ligand-Target Pair
Affinity DataIC50: 1.11E+4nMAssay Description:The fluorescence peptide cleavage assay was performed in a 96-well plate in which each reaction mixture contained MAPKKide, LF (List Biological Labor...More data for this Ligand-Target Pair
Affinity DataIC50: 1.18E+4nMAssay Description:The fluorescence peptide cleavage assay was performed in a 96-well plate in which each reaction mixture contained MAPKKide, LF (List Biological Labor...More data for this Ligand-Target Pair
Affinity DataIC50: 1.18E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+4nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMpH: 7.4 T: 2°CAssay Description:Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont...More data for this Ligand-Target Pair
Affinity DataIC50: 1.35E+4nMpH: 7.4 T: 2°CAssay Description:Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont...More data for this Ligand-Target Pair
Affinity DataIC50: 1.43E+4nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 1.45E+4nMpH: 7.4 T: 2°CAssay Description:Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair



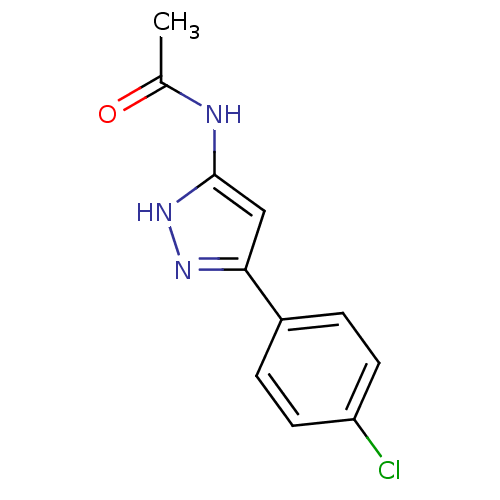
 3D Structure (crystal)
3D Structure (crystal)