BDBM50521385 CHEMBL4580164
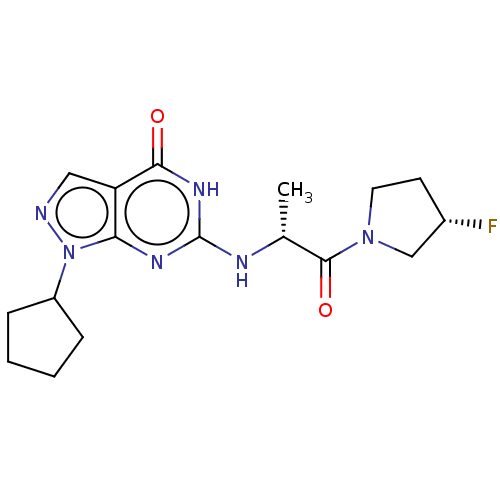
SMILES C[C@@H](Nc1nc2n(ncc2c(=O)[nH]1)C1CCCC1)C(=O)N1CC[C@H](F)C1
InChI Key InChIKey=HOQGZKUBNCAZBE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 16 hits for monomerid = 50521385
Found 16 hits for monomerid = 50521385
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Human)
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 8.70nMAssay Description:Inhibition of recombinant human PDE9A2 catalytic domain (181 to 506 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human PDE4D2 catalytic domain (86 to 413 residues) using [3H]cAMP as substrate after 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.01E+3nMAssay Description:Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A(Human)
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PDE8A1 catalytic domain (480 to 820 residues) (unknown origin) using [3H]-cAMP as substrate after 15 mins by liquid scintillation count...More data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Human)
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged PDE1C (2 to 634 residues) expressed in baculovirus infected Sf9 cells using 3H-cGMP as substrat...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Human)
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 7.31E+3nMAssay Description:Inhibition of human PDE10A catalytic domain (449 to 770 residues) using [3H]-cAMP as substrate after 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP1A2 in human liver microsomes using [14C]-ethoxyresorufin as substrate preincubated for 2 mins in presence of NADPH followed by subs...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2B6 in human liver microsomes in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using [14C]-erythromycin as substrate preincubated for 2 mins in presence of NADPH followed by substra...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG expressed in CHO cells by patch clamp assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes using [14C]-naproxen as substrate preincubated for 2 mins in presence of NADPH followed by substrate a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PDE2A catalytic domain (580 to 919 residues) (unknown origin) using 3H-cGMP as substrate after 15 mins by liquid scintillation counting...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using [14C]-dextromethorphan as substrate preincubated for 2 mins in presence of NADPH followed by sub...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A(Human)
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 4.83E+3nMAssay Description:Inhibition of PDE7A1 catalytic domain (130 to 482 residues) (unknown origin) using [3H]-cAMP as substrate after 15 mins by liquid scintillation count...More data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B(Human)
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of PDE1B catalytic domain (10 to 487 residues) (unknown origin) using 3H-cGMP as substrate after 15 mins by liquid scintillation counting ...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Human)
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PDE3A catalytic domain (679 to 1087 residues) (unknown origin) using 3H-cAMP as substrate after 15 mins by liquid scintillation countin...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)