BDBM50517586 CHEMBL4562603
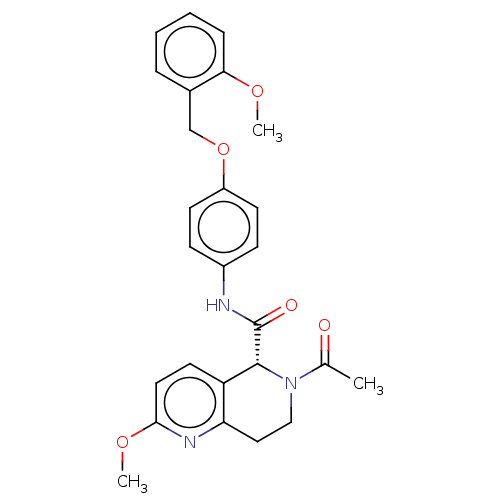
SMILES COc1ccc2[C@@H](N(CCc2n1)C(C)=O)C(=O)Nc1ccc(OCc2ccccc2OC)cc1
InChI Key InChIKey=RNCVXSNHCXEILI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50517586
Found 3 hits for monomerid = 50517586
Affinity DataEC50: 8.10E+3nMAssay Description:Agonist activity at RORgammaT (unknown origin) expressed in human Jurkat cells co-expressing human IL-17 ROR response element by luciferase reporter ...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Displacement of BODIPY-labeled-5-((2Z)-2-((1-(difluoroboryl)-3,5-dimethyl-1H-pyrrol-2-yl)-methylene)-2H-pyrrol-5-yl)-N-(2-((3,5-difluoro-4-(trimethyl...More data for this Ligand-Target Pair
Affinity DataEC50: 53nMAssay Description:Agonist activity at His-tagged human RORgammaT assessed as induction of biotinylated SRC-1 peptide recruitment after 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)