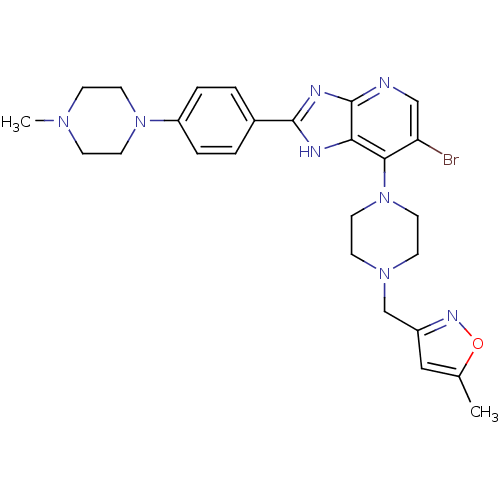
BDBM50335609 3-((4-(6-bromo-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)piperazin-1-yl)methyl)-5-methylisoxazole::6-BROMO-7-{4-[(5-METHYLISOXAZOL-3-YL)METHYL]PIPERAZIN-1-YL}-2-[4-(4-METHYLPIPERAZIN-1-YL)PHENYL]-1H-IMIDAZO[4,5-B]PYRIDINE::CHEMBL1236904
SMILES CN1CCN(CC1)c1ccc(cc1)-c1nc2ncc(Br)c(N3CCN(Cc4cc(C)on4)CC3)c2[nH]1
InChI Key InChIKey=GFLQCBTXTRCREJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50335609
Found 4 hits for monomerid = 50335609
Affinity DataIC50: 15nMAssay Description:Inhibition of Aurora AMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Human)
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataKd: 1.20nMAssay Description:Binding affinity to FLT3More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human ERG by whole-cell voltage clampingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair