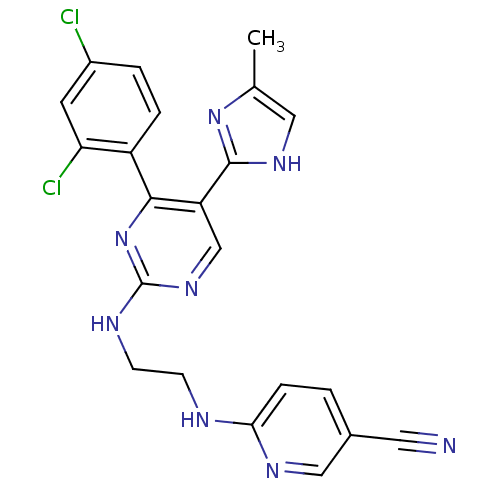
BDBM50325983 6-(2-(4-(2,4-dichlorophenyl)-5-(4-methyl-1H-imidazol-2-yl)pyrimidin-2-ylamino)ethylamino)nicotinonitrile::CHEMBL412142::CHIR-99021::CT 99021::US11203601, Compound Table 1.19
SMILES Cc1c[nH]c(n1)-c1cnc(NCCNc2ccc(cn2)C#N)nc1-c1ccc(Cl)cc1Cl
InChI Key InChIKey=AQGNHMOJWBZFQQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 29 hits for monomerid = 50325983
Found 29 hits for monomerid = 50325983
TargetVascular endothelial growth factor receptor 2(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human KDR using KDRY1175 [B91616] biotin-GGGGQDGKDYIVLPI-NH2 peptide substrate after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human wildtype full length GSK-3betaMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of N-terminal His6-tagged GSK-3 beta (unknown origin) expressed in Rosetta 2DE3 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of GSK-3beta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:Inhibition of from NanoLuc-fused GSK-3-beta (unknown origin) transfected in HEK293 cells using tracer K8 incubated for 1 hr by NanoBRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:A selection of compounds was screened against a selected panel of kinases based on single point inhibitory ability to determine absolute inhibitory a...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:A selection of compounds was screened against a selected panel of kinases based on single point inhibitory ability to determine absolute inhibitory a...More data for this Ligand-Target Pair
Affinity DataIC50: 9.90nMAssay Description:Inhibition of human recombinant GSK3beta using GSK3 peptide as substrate preincubated for 15 mins followed by ATP addition and measured after 90 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of GSK3beta (unknown origin)More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 1(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human FLT1 using KDRY1175 [B91616] biotin-GGGGQDGKDYIVLPI-NH2 peptide substrate after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human Tie2 using biotin-GGGGAPDLYKDFLT peptide substrate after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human AKT1/PKB using phospho-AKT peptide substrate after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human PKCzeta using biotin-PKC-86 peptide substrate after 30 mins in the presence of phosphatidylserine and diacylglycerol activators b...More data for this Ligand-Target Pair
TargetInsulin-like growth factor 1 receptor(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human IGF1 receptor tyrosine kinase using biotin-GGGGKKKSPGEYVNIEFG-amide peptide substrate after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
TargetFibroblast growth factor receptor 1(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human bFGF receptor tyrosine kinase using KDRY1175 [B91616] biotin-GGGGQDGKDYIVLPI-NH2 peptide substrate after 1 hr by scintillation co...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 1(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERK2 using myelin basic protein substrate after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.80E+3nMAssay Description:Inhibition of human CDC2 using biotin histone H1 peptide substrate after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.80nMAssay Description:Inhibition of human GSK3alpha using biotin-CREB peptide substrate after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
TargetCasein kinase I isoform epsilon(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human CK1epsilon using biotin peptide substrate after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human CHK1 using biotin-CDC25 peptide substrate after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Target3-phosphoinositide-dependent protein kinase 1(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human PDK1 using unactivated AKT substrate in presence of COPC, DOPS and PIP3 activators after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human insulin receptor tyrosine kinase after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:Inhibition of human GSK3beta using biotin-CREB peptide substrate after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Human)
The Rockefeller University
Curated by ChEMBL
The Rockefeller University
Curated by ChEMBL
Affinity DataIC50: 8.80E+3nMAssay Description:Inhibition of CDK1/cyclinBMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of GSK3alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of GSK3-betaMore data for this Ligand-Target Pair
Affinity DataEC50: 1.50E+3nMAssay Description:Inhibition of GSK3-beta in human HepG2 cells assessed as incorporation of [3H]glucose into glycogen after 3 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of GSK3-betaMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)