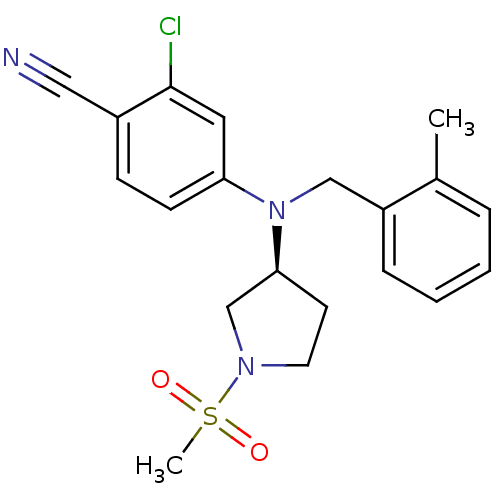
BDBM50304974 (S)-2-chloro-4-((2-methylbenzyl)(1-(methylsulfonyl)pyrrolidin-3-yl)amino)benzonitrile::2-chloro-4-{(2-methylbenzyl)[(3S)-1-(methylsulfonyl)pyrrolidin-3-yl]amino}benzonitrile::CHEMBL601500
SMILES Cc1ccccc1CN([C@H]1CCN(C1)S(C)(=O)=O)c1ccc(C#N)c(Cl)c1
InChI Key InChIKey=OTRAFCFYTZJLKH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50304974
Found 5 hits for monomerid = 50304974
Affinity DataEC50: 25nMAssay Description:Agonist activity at PR in human T47D cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of human ERG by patch clamp techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair