BDBM50259973 CHEMBL4098403
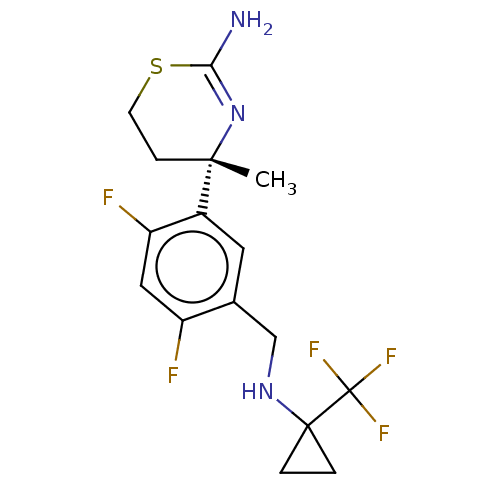
SMILES C[C@]1(CCSC(N)=N1)c1cc(CNC2(CC2)C(F)(F)F)c(F)cc1F
InChI Key InChIKey=XKONRMXLBXCJAM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50259973
Found 6 hits for monomerid = 50259973
Affinity DataIC50: 157nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 405nMAssay Description:Inhibition of BACE2 (unknown origin) by cell free assayMore data for this Ligand-Target Pair
Affinity DataIC50: 69nMAssay Description:Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass...More data for this Ligand-Target Pair
Affinity DataIC50: 3.24E+4nMAssay Description:Inhibition of Cathepsin D (unknown origin) by cell free assayMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of human ERG expressed in CHO cells at -80 mV holding potential after 5 mins by patch clamp assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)