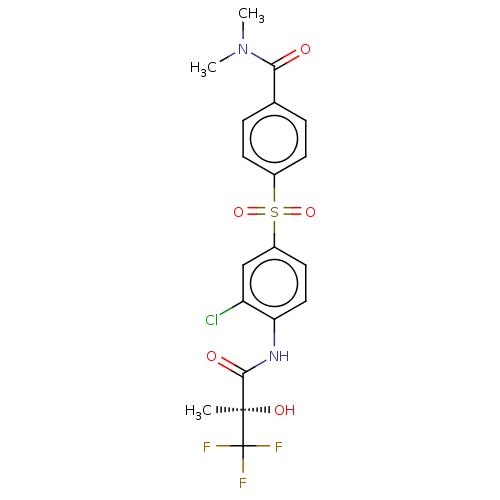
BDBM50236535 CHEMBL1231132
SMILES CN(C)C(=O)c1ccc(cc1)S(=O)(=O)c1ccc(NC(=O)[C@@](C)(O)C(F)(F)F)c(Cl)c1
InChI Key InChIKey=DTDZLJHKVNTQGZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50236535
Found 5 hits for monomerid = 50236535
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial(Human)
Vernalis (R&D)
Curated by ChEMBL
Vernalis (R&D)
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Inhibition of PDHK1 in human PC3 cells assessed as decrease in phosphorylation of E1alpha subunit at serine 293 residue after 1 hr by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.29E+4nMAssay Description:Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Human)
Capital Medical University
Curated by ChEMBL
Capital Medical University
Curated by ChEMBL
Affinity DataEC50: 80nMAssay Description:Inhibition of PDK2 (unknown origin)More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Human)
Capital Medical University
Curated by ChEMBL
Capital Medical University
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of E2-activated human PDHK2More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Human)
Capital Medical University
Curated by ChEMBL
Capital Medical University
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of E2-activated human PDHK2More data for this Ligand-Target Pair