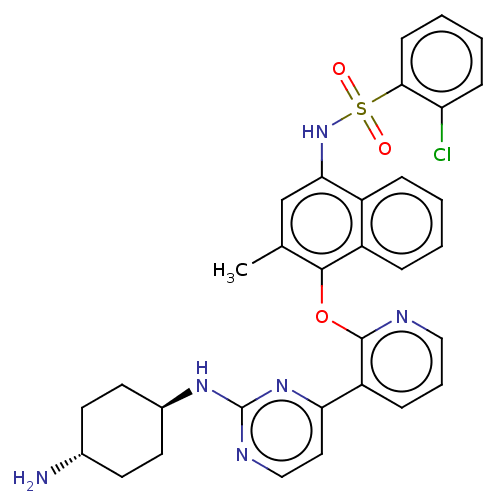
BDBM50043710 CHEMBL3356007
SMILES Cc1cc(NS(=O)(=O)c2ccccc2Cl)c2ccccc2c1Oc1ncccc1-c1ccnc(N[C@H]2CC[C@H](N)CC2)n1
InChI Key InChIKey=VMXTWHCDROGRNY-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50043710
Found 4 hits for monomerid = 50043710
Affinity DataIC50: 14nMAssay Description:Inhibition of human IRE1alpha using 5'[6FAM]-GAGUCCGCAGCACUC-[BHQ1]3' substrate by biochemical fluorescence quenching assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of human wild type JNK3 using biotinylated ATF2 substrate assessed as phosphorylation at thr53 on ATF2 by fluorescent plate readerMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:Competitive inhibition of IRE1alpha LKR domain (Q470 to L997 residues) (unknown origin) expressed in Sf9 insect cells using mini-XBP-1 stem-loop RNA ...More data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:Inhibition of IRE1alpha (unknown origin) assessed as XBP-1 slicing luciferase activity incubated for 1 hr followed by stimulation with thapsigargin m...More data for this Ligand-Target Pair