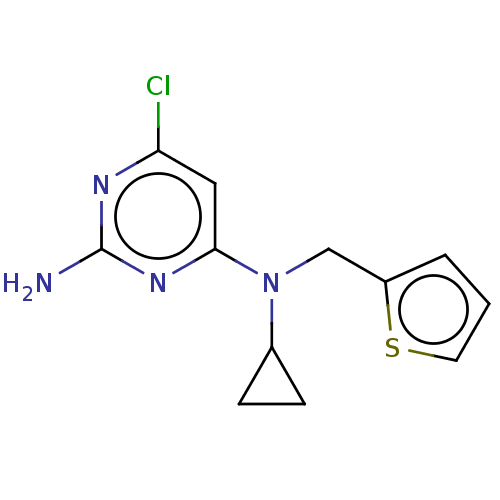
BDBM492902 US10981899, Example RU-0204277-LRE1
SMILES Nc1nc(Cl)cc(n1)N(Cc1cccs1)C1CC1
InChI Key InChIKey=PDWZXKSZLRVSEH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 492902
Found 3 hits for monomerid = 492902
Affinity DataIC50: 3.30E+3nMAssay Description:The RapidFire 365 High-throughput MS System (Agilent Technologies; RF-MSS) can process samples every 15 seconds allowing analysis of a 384 well plate...More data for this Ligand-Target Pair
Affinity DataIC50: 6.30E+3nMAssay Description:INS-1E insulinoma cells were incubated in 2.5 mM glucose Krebs-Ringer buffer (pH 7.5) supplemented with 2 mM sodium bicarbonate, 10 mM HEPES, and 0.1...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)