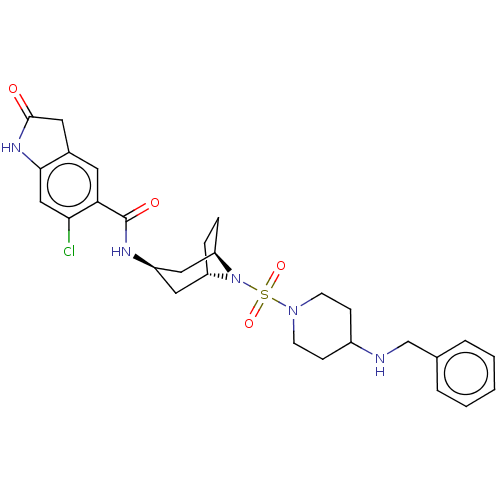
BDBM378459 N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)sulfonyl)-8-azabicyclo[3.2.1]octan-3-yl)-6-chloro-2-oxoindoline-5-carboxamide::US10266526, Compound 592
SMILES Clc1cc2NC(=O)Cc2cc1C(=O)N[C@@H]1C[C@@H]2CC[C@H](C1)N2S(=O)(=O)N1CCC(CC1)NCc1ccccc1
InChI Key InChIKey=SSDPURPCLGSTBN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 378459
Found 6 hits for monomerid = 378459
Affinity DataIC50: 2.58nMAssay Description:The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use....More data for this Ligand-Target Pair
Affinity DataIC50: 48nMAssay Description:Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of SMYD2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Non-competitive inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using varyin...More data for this Ligand-Target Pair
Affinity DataKi: 4.70nMAssay Description:Mixed type inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using fixed N-ter...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)