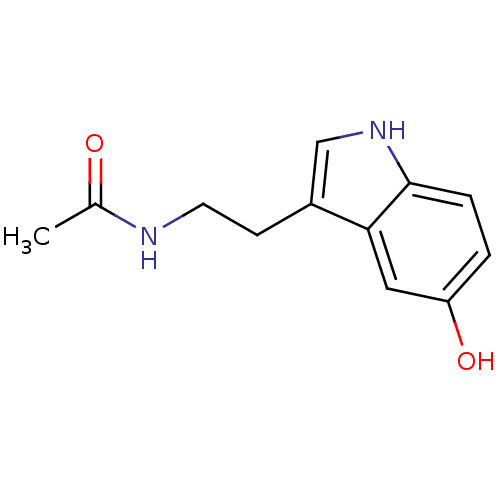
BDBM29612 CHEMBL33103::CVD-0001578::JOH-MSK-a63bdd1d-4::N-ACETYL SEROTONIN::N-Acetyl-5-hydroxytryptamine::N-Acetyltryptamine,5-Hydroxy::N-acetylserotonin::Normelatonin::Serotonin,N-acetyl
SMILES CC(=O)NCCc1c[nH]c2ccc(O)cc12
InChI Key InChIKey=MVAWJSIDNICKHF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 23 hits for monomerid = 29612
Found 23 hits for monomerid = 29612
TargetMelatonin receptor type 1A(Human)
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
TargetMelatonin receptor type 1B(Human)
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
TargetMelatonin receptor type 1B(Human)
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
TargetMelatonin receptor type 1B(Human)
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
TargetMelatonin receptor type 1A(Human)
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
TargetMelatonin receptor type 1B(Human)
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
TargetMelatonin receptor type 1B(Human)
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
TargetMelatonin receptor type 1A(Pig)
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
TargetMelatonin receptor type 1A(Pig)
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
TargetMelatonin receptor type 1A(Human)
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
Affinity DataKi: 1.64E+3nMAssay Description:In vitro binding affinity against melatonin receptor using 2-[125I]iodomelatonin (0.05 nM) and chicken brain membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human sepiapterin reductase using L-sepiapterin as substrate preincubated for 15 mins followed by substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 9.90E+3nMpH: 8.0 T: 2°CAssay Description:The activity of recombinant human QR2 under steady-state conditions was evaluated on a MolecularDevices SpectraMax Plus 384 UV-visible spectrophotome...More data for this Ligand-Target Pair
Affinity DataEC50: 1.96E+4nMAssay Description:Positive allosteric modulation of recombinant human IDOL transfected in mouse P1.HTR cells assessed as increase in Kyn production incubated for 24 hr...More data for this Ligand-Target Pair
Affinity DataEC50: 2.10E+4nMAssay Description:Positive allosteric modulation of recombinant mouse IDOL transfected in mouse P1.HTR cells assessed as increase in Kyn production incubated for 24 hr...More data for this Ligand-Target Pair
Affinity DataIC50: 9.36E+4nMAssay Description:Inhibition of human Notum using OPTS substrate by fluorescence based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9.90E+4nMAssay Description:The assay was performed according to the published procedure. Briefly, compounds were seeded into assay-ready plates (Greiner 384PP, cat# 781280) usi...More data for this Ligand-Target Pair
Affinity DataIC50: 9.95E+4nMAssay Description:Compounds were seeded into assay-ready plates (Greiner 384 low volume, cat 784900) using an Echo 555 acoustic dispenser, and DMSO was back-filled for...More data for this Ligand-Target Pair
Affinity DataIC50: 9.95E+4nMAssay Description:Compounds were seeded into assay-ready plates (Greiner 384 low volume, cat. no. 784900) using an Echo 555 acoustic dispenser, and dimethyl sulfoxide ...More data for this Ligand-Target Pair
TargetTyrosinase(Mouse)
National Institute of Advanced Industrial Science and Technology
Curated by ChEMBL
National Institute of Advanced Industrial Science and Technology
Curated by ChEMBL
Affinity DataKi: 2.40E+5nMAssay Description:Apparent noncompetitive inhibition of catecholase activity of tyrosinase in mouse B16 cells by Lineweaver-Burke plot analysisMore data for this Ligand-Target Pair
TargetTyrosinase(Mouse)
National Institute of Advanced Industrial Science and Technology
Curated by ChEMBL
National Institute of Advanced Industrial Science and Technology
Curated by ChEMBL
Affinity DataIC50: 3.11E+5nMAssay Description:Inhibition of catecholase activity of tyrosinase in mouse B16 cells assessed as dopachrome formationMore data for this Ligand-Target Pair