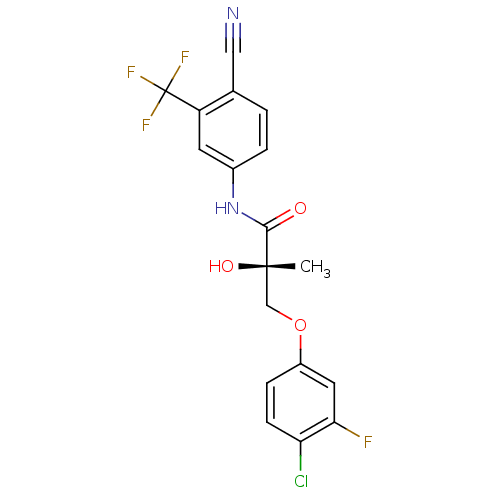
BDBM26261 (2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide::C-31::CHEMBL512283::US9278914, S-II
SMILES C[C@](O)(COc1ccc(Cl)c(F)c1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F
InChI Key InChIKey=SSFVOEAXHZGTRJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 26261
Found 7 hits for monomerid = 26261
Affinity DataIC50: 2.00E+4nMT: 2°CAssay Description:P450 enzyme inhibition was measured using human cDNA-expressed CYP3A4, 2D6, 2C19, 2C9, and 1A2 recombinant enzymes and fluorogenic substrates (coumar...More data for this Ligand-Target Pair
Affinity DataIC50: 1.77E+4nMT: 2°CAssay Description:P450 enzyme inhibition was measured using human cDNA-expressed CYP3A4, 2D6, 2C19, 2C9, and 1A2 recombinant enzymes and fluorogenic substrates (coumar...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMT: 2°CAssay Description:P450 enzyme inhibition was measured using human cDNA-expressed CYP3A4, 2D6, 2C19, 2C9, and 1A2 recombinant enzymes and fluorogenic substrates (coumar...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMT: 2°CAssay Description:P450 enzyme inhibition was measured using human cDNA-expressed CYP3A4, 2D6, 2C19, 2C9, and 1A2 recombinant enzymes and fluorogenic substrates (coumar...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMT: 2°CAssay Description:P450 enzyme inhibition was measured using human cDNA-expressed CYP3A4, 2D6, 2C19, 2C9, and 1A2 recombinant enzymes and fluorogenic substrates (coumar...More data for this Ligand-Target Pair
Affinity DataKi: 1.70nM ΔG°: -11.1kcal/molepH: 7.4 T: 2°CAssay Description:The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (...More data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Agonist activity at androgen receptorMore data for this Ligand-Target Pair