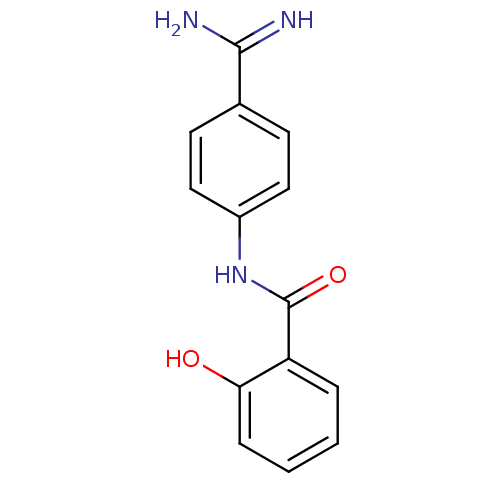
BDBM14155 APC-7136::CHEMBL64708::N-(4-CARBAMIMIDOYL-PHENYL)-2-HYDROXY-BENZAMIDE::N-(4-carbamimidoylphenyl)-2-hydroxybenzamide
SMILES NC(=N)c1ccc(NC(=O)c2ccccc2O)cc1
InChI Key InChIKey=MYHDBRFFPZXDMX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 14155
Found 13 hits for monomerid = 14155
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of recombinant human N-terminal his-tagged KLK1 (19 to 262 residues) expressed in baculovirus infected sf9 insect cells using R110 labelle...More data for this Ligand-Target Pair
Affinity DataIC50: 6.31E+3nMAssay Description:Inhibition of recombinant human FLAG-tagged KLK5 expressed in baculovirus infected sf9 insect cells using (Tos-Gly-Pro-Arg)2[R110].2TFA as substrate ...More data for this Ligand-Target Pair
Affinity DataKi: 2.70E+3nMAssay Description:Inhibition of urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
Affinity DataKi: 3.20E+3nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 3.80E+3nM ΔG°: -7.31kcal/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 6.60E+3nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Activation of tissue plasminogenMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:The compound was evaluated to inhibit the Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 2.20E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+4nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 2.40E+4nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 9.00E+4nMAssay Description:Inhibition of plasminMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)