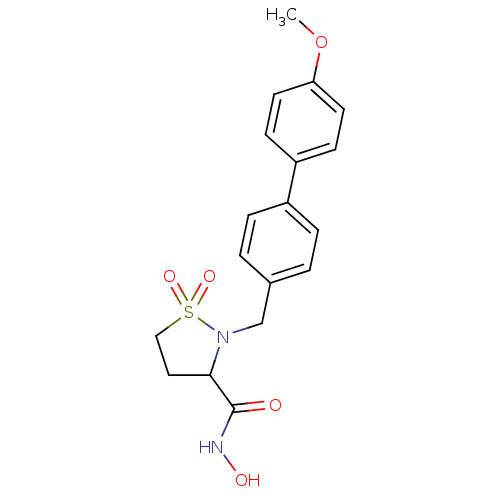
BDBM11548 CHEMBL100570::N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)methyl]isothiazolidine-3-carboxamide 1,1-dioxide::N-hydroxy-2-{[4-(4-methoxyphenyl)phenyl]methyl}-1,1-dioxo-1,2-thiazolidine-3-carboxamide::Sultam Hydroxamate 15a
SMILES COc1ccc(cc1)-c1ccc(CN2C(CCS2(=O)=O)C(=O)NO)cc1
InChI Key InChIKey=RNCWXNOSFWYLTA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 11548
Found 17 hits for monomerid = 11548
TargetDisintegrin and metalloproteinase domain-containing protein 17(Human)
The M. S. University of Baroda
Curated by ChEMBL
The M. S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of TACEMore data for this Ligand-Target Pair
TargetDisintegrin and metalloproteinase domain-containing protein 17(Human)
The M. S. University of Baroda
Curated by ChEMBL
The M. S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity against TACEMore data for this Ligand-Target Pair
Affinity DataKi: 3.80nMAssay Description:The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m...More data for this Ligand-Target Pair
Affinity DataKi: 3.80nMAssay Description:Inhibition of MMP-2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3.80nMAssay Description:Binding affinity to MMP2More data for this Ligand-Target Pair
Affinity DataKi: 46.7nMAssay Description:The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m...More data for this Ligand-Target Pair
Affinity DataKi: 47nMAssay Description:Binding affinity to MMP9More data for this Ligand-Target Pair
Affinity DataKi: 47nMAssay Description:Inhibition of MMP-9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:Binding affinity to MMP13More data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m...More data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:Inhibition of MMP-13 (unknown origin)More data for this Ligand-Target Pair
TargetInterstitial collagenase(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: >4.95E+3nMAssay Description:Binding affinity to MMP1More data for this Ligand-Target Pair
TargetInterstitial collagenase(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 4.95E+3nMAssay Description:Inhibition of MMP-1 (unknown origin)More data for this Ligand-Target Pair
TargetInterstitial collagenase(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: >4.95E+3nM ΔG°: >-7.23kcal/molepH: 7.5 T: 2°CAssay Description:The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m...More data for this Ligand-Target Pair