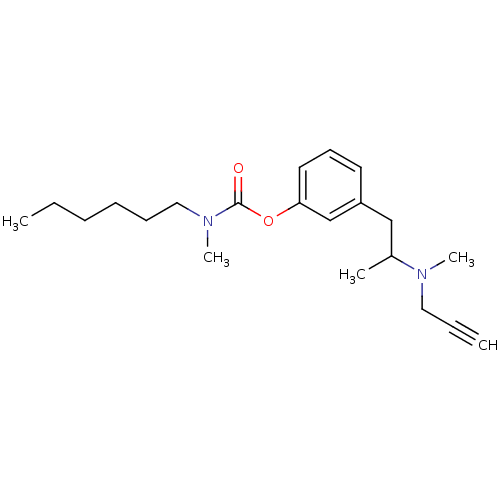
BDBM10826 3-{2-[methyl(prop-2-yn-1-yl)amino]propyl}phenyl N-hexyl-N-methylcarbamate::CHEMBL356385::Phenethylamine deriv. 53d
SMILES CCCCCCN(C)C(=O)Oc1cccc(CC(C)N(C)CC#C)c1
InChI Key InChIKey=YNBUJXJHKVCTQX-UHFFFAOYSA-N
Data 13 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 10826
Found 13 hits for monomerid = 10826
Affinity DataIC50: 3.06E+3nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 720nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.06E+3nMAssay Description:Inhibition of human AchEMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of MAOBMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of MAOAMore data for this Ligand-Target Pair
Affinity DataIC50: 720nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated 60 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.06E+3nMAssay Description:Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate preincubated for 60 mins followed by substrate addition by ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate preincubated for 60 mins followed by substrate addition by ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of rat brain MAO-A using 14C-5-hydroxytryptamine creatinine disulfate as substrate preincubated for 60 mins followed by substrate addition...More data for this Ligand-Target Pair
Affinity DataIC50: 3.06E+3nMAssay Description:Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate preincubated for 60 mins followed by substrate addition by ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of rat brain MAO-B using 14C-phenylethylamine as substrate preincubated for 60 mins followed by substrate addition and measured after 20 m...More data for this Ligand-Target Pair