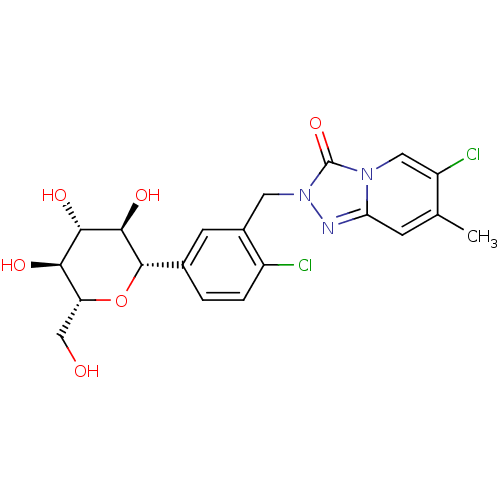
BDBM104500 US8575114, I-12
SMILES Cc1cc2nn(Cc3cc(ccc3Cl)[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c(=O)n2cc1Cl
InChI Key InChIKey=LIKSGJKEDNKMAF-UHFFFAOYSA-N
Data 10 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 104500
Found 10 hits for monomerid = 104500
Affinity DataIC50: 36.6nMAssay Description:The inhibitory activity of the compounds of this invention against the SGLT-1 or SGLT-2 transporters.More data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+4nMAssay Description:The inhibitory activity of the compounds of this invention against the SGLT-1 or SGLT-2 transporters.More data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Inhibition of human SGLT2 expressed in CHOK1 cells assessed as inhibition of [14C]-AMG uptake after 3 hrs by microbeta scintillation counting analysi...More data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+4nMAssay Description:Inhibition of human SGLT1 expressed in CHOK1 cells assessed as inhibition of [14C]-AMG uptake after 3 hrs by microbeta scintillation counting analysi...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of CYP2B6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair