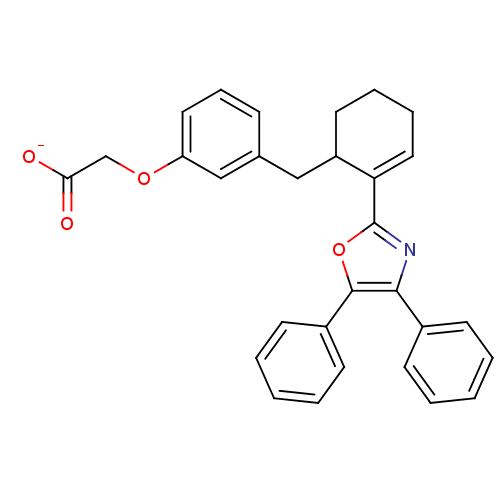
BDBM50136234 CHEMBL132589::FR-181157::Sodium; {3-[(R)-2-(4,5-diphenyl-oxazol-2-yl)-cyclohex-2-enylmethyl]-phenoxy}-acetate::Sodium; {3-[(S)-2-(4,5-diphenyl-oxazol-2-yl)-cyclohex-2-enylmethyl]-phenoxy}-acetate::Sodium; {3-[2-(4,5-diphenyl-oxazol-2-yl)-cyclohex-2-enylmethyl]-phenoxy}-acetate::sodium (S)-2-(3-((2-(4,5-diphenyloxazol-2-yl)cyclohex-2-enyl)methyl)phenoxy)acetate
SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1
InChI Key InChIKey=QVZSFHJAGXKMMQ-UHFFFAOYSA-M
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 20 hits for monomerid = 50136234
Found 20 hits for monomerid = 50136234
Affinity DataIC50: 60nMAssay Description:Agonist activity at human PGI2 receptor assessed as inhibition of ADP-induced platelet aggregationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Agonist activity at rat PGI2 receptor assessed as inhibition of ADP-induced platelet aggregationMore data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:In vitro Prostacyclin (PGI-2) receptor binding assay was determined based on displacement of [3H]iloprost radioligand from cloned human IP receptorMore data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Inhibition of [3H]iloprost binding to human prostanoid IP receptorMore data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Inhibition of [3H]-Iloprost binding to human Prostanoid IP receptorMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Displacement of [3H]iloprost from cloned human PGI2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]-PGE-2 binding to human prostanoid EP1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]PGE-2 binding to Prostanoid EP1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]-PGF-2 binding to human Prostanoid FP receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]PGD-2 binding to human Prostanoid DP receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]PGE-2 binding to Prostanoid EP2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]SQ-29,548 binding to human Prostanoid TP receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]PGF-2 binding to human prostanoid FP receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]PGE-2 binding to human prostanoid EP2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]PGD-2 binding to human prostanoid DP receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]SQ-29,548 binding to human prostanoid TP receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]PGE-2 binding to human prostanoid EP3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.02E+3nMAssay Description:Inhibition of [3H]PGE-2 binding to Prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.02E+3nMAssay Description:Inhibition of [3H]PGE-2 binding to human prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.80E+3nMAssay Description:Inhibition of [3H]-PGE-2 binding to Prostanoid EP3 receptorMore data for this Ligand-Target Pair