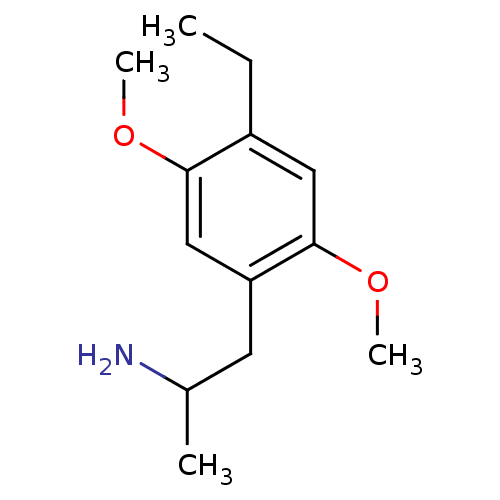
BDBM81965 1-(4-ethyl-2,5-dimethoxyphenyl)propan-2-amine::CAS_62066::CHEMBL8224::DOET,(-)::NSC_62066::doet
SMILES CCc1cc(OC)c(CC(C)N)cc1OC
InChI Key InChIKey=HXJKWPGVENNMCC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 53 hits for monomerid = 81965
Found 53 hits for monomerid = 81965
Affinity DataEC50: 9.18nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
Affinity DataEC50: 2.40nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
Affinity DataKi: 14.4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 27.3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 28.8nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2 receptor from frontal cortical regions of male Sprague-Dawley rat homogenates, using [3H]ketanserin as...More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Displacement of [3H]ketanserin from 5HT2A receptor in Sprague-Dawley rat brain by liquid scintillation spectroscopyMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Binding affinity to rat cortical membranes at 5-hydroxytryptamine 2 (5-HT2) receptor using [3H]KET as a radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Binding affinity against 5-hydroxytryptamine 2 receptor of rat frontal cortex using [3H]ketanserin radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 101nMAssay Description:Compound was tested for binding affinity towards 5-HT1C (5-HT1C) receptor from frontal cortical regions of male Sprague-Dawley rat homogenates, using...More data for this Ligand-Target Pair
Affinity DataKi: 107nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 574nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 1.23E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 1.28E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 1.45E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 2.20E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 3.55E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 4.57E+3nMAssay Description:Evaluated for binding affinity towards rat cortical membranes at 5-hydroxytryptamine 1 receptor binding site by using [3H]-5-HT as a radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 5.72E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 6.62E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 9.56E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 9.78E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair