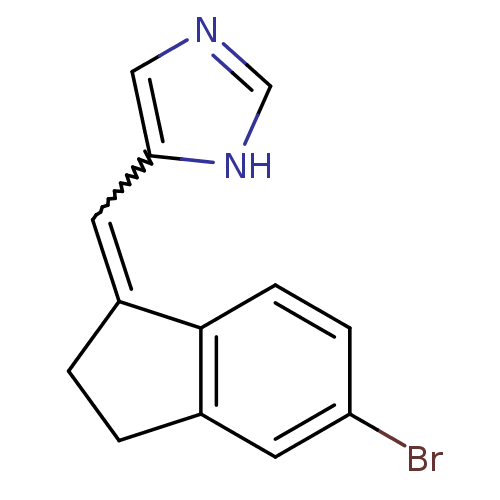
BDBM8883 5-[(E)-(5-Bromo-2,3-dihydro-1H-inden-1-ylidene)methyl]-1H-imidazole::5-{[(1E)-5-bromo-2,3-dihydro-1H-inden-1-ylidene]methyl}-1H-imidazole::Imidazolylmethyleneindane 9a::Imidazolylmethyleneindane 9b
SMILES Brc1ccc2C(CCc2c1)=Cc1cnc[nH]1
InChI Key InChIKey=SNWLVVNOSVKKRA-UHFFFAOYSA-N
Data 6 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 8883
Found 8 hits for monomerid = 8883
Affinity DataIC50: 26.2nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 23.5nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 92.8nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 10.3nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair