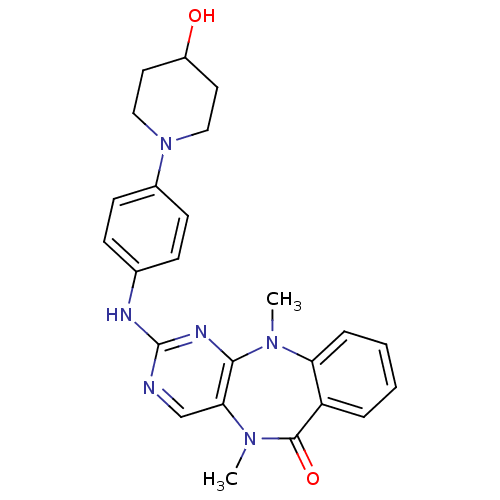
BDBM81552 Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1::Scaffold, B54
SMILES CN1c2ccccc2C(=O)N(C)c2cnc(Nc3ccc(cc3)N3CCC(O)CC3)nc12
InChI Key InChIKey=DFQAJLQXPSPNJE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 81552
Found 8 hits for monomerid = 81552
Affinity DataIC50: 8nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:In vitro kinase assay of Aurora A, B, and C using Z-LYTE technology (Invitrogen) and ATP at Km apparent for each kinase.More data for this Ligand-Target Pair
Affinity DataIC50: 18.4nMAssay Description:In vitro kinase assay of Aurora A, B, and C using Z-LYTE technology (Invitrogen) and ATP at Km apparent for each kinase.More data for this Ligand-Target Pair
Affinity DataIC50: 24.6nMAssay Description:In vitro kinase assay of Aurora A, B, and C using Z-LYTE technology (Invitrogen) and ATP at Km apparent for each kinase.More data for this Ligand-Target Pair
Affinity DataIC50: 109nMAssay Description:Inhibition of recombinant human N-terminal His6-tagged DCLK1 (G351 to H689 residues) expressed in Escherichia coli BL21 DE3 using 5-FAM-KKLRRTLSVA-CO...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant human GST-tagged LRRK2 catalytic domain (970 to 2527 residues) expressed in baculovirus expression system using LRRKtide as...More data for this Ligand-Target Pair