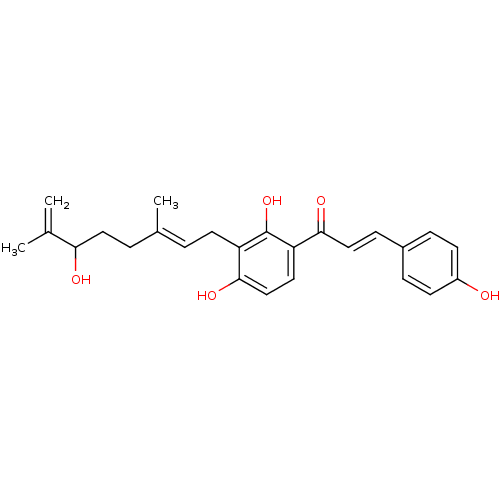
BDBM76798 (E)-1-[2,4-dihydroxy-3-[(2E)-6-hydroxy-3,7-dimethyl-octa-2,7-dienyl]phenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one::(E)-1-[2,4-dihydroxy-3-[(2E)-6-hydroxy-3,7-dimethylocta-2,7-dienyl]phenyl]-3-(4-hydroxyphenyl)-2-propen-1-one::(E)-1-[2,4-dihydroxy-3-[(2E)-6-hydroxy-3,7-dimethylocta-2,7-dienyl]phenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one::(E)-1-[3-[(2E)-3,7-dimethyl-6-oxidanyl-octa-2,7-dienyl]-2,4-bis(oxidanyl)phenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one::MLS002472953::SMR001397061::cid_10409180::xanthoangelol B
SMILES CC(=C)C(O)CC\C(C)=C\Cc1c(O)ccc(C(=O)\C=C\c2ccc(O)cc2)c1O
InChI Key InChIKey=NCHZAFAGBAEJJJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 76798
Found 8 hits for monomerid = 76798
TargetSentrin-specific protease 6(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 4.22E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 2.81E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetSentrin-specific protease 8(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 5.08E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetNeuraminidase(Influenza A virus (strain A/Brevig Mission/1/1918 ...)
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Affinity DataIC50: 2.25E+4nMAssay Description:Inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid ...More data for this Ligand-Target Pair
TargetSensory histidine kinase DcuS(Staphylococcus aureus)
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataIC50: 3.39E+5nMAssay Description:Inhibition of recombinant Staphylococcus aureus AgrC autophosphorylation expressed in Escherichia coli BL21(DE3) preincubated for 15 mins followed by...More data for this Ligand-Target Pair
TargetHistidine protein kinase SaeS(Staphylococcus aureus (strain NCTC 8325 / PS 47))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataIC50: 2.20E+5nMAssay Description:Inhibition of recombinant Staphylococcus aureus SaeS autophosphorylation expressed in Escherichia coli BL21(DE3) preincubated for 15 mins followed by...More data for this Ligand-Target Pair
TargetHistidine protein kinase SaeS(Staphylococcus aureus (strain NCTC 8325 / PS 47))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKd: 4.00E+4nMAssay Description:Binding affinity to Staphylococcus aureus SaeS kinase domain after 15 mins by fluorescence spectrophotometryMore data for this Ligand-Target Pair
TargetNeuraminidase(Influenza A virus (strain A/Brevig Mission/1/1918 ...)
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Affinity DataKi: 2.07E+4nMAssay Description:Noncompetitive inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetyln...More data for this Ligand-Target Pair