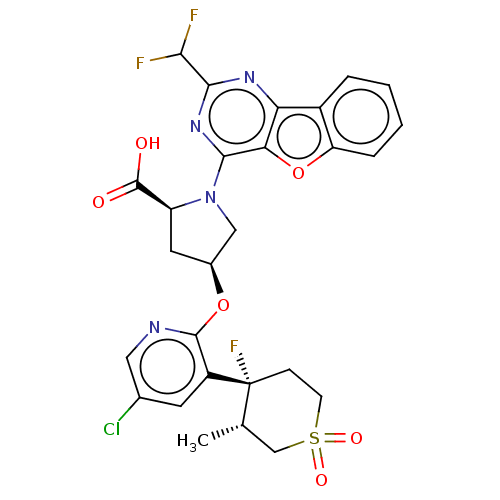
BDBM685859 US12043625, Ex. 1.13::US12043625, Example 1.13
SMILES C[C@H]1CS(=O)(=O)CC[C@]1(F)c1cc(Cl)cnc1O[C@H]1C[C@H](N(C1)c1nc(nc2c3ccccc3oc12)C(F)F)C(O)=O
InChI Key InChIKey=QAOBFNWSNRYRCD-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 685859
Found 3 hits for monomerid = 685859
Affinity DataIC50: 2nMAssay Description:PKC-theta and PKC-delta biochemical activities were measured using the PKC-theta HTRF KinEASEkit kit, according to manufacturer's instructions (C...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:PKC-theta and PKC-delta biochemical activities were measured using the PKC-theta HTRF KinEASEkit kit, according to manufacturer's instructions (C...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:in vitro human biochemical cGAS inhibition.More data for this Ligand-Target Pair