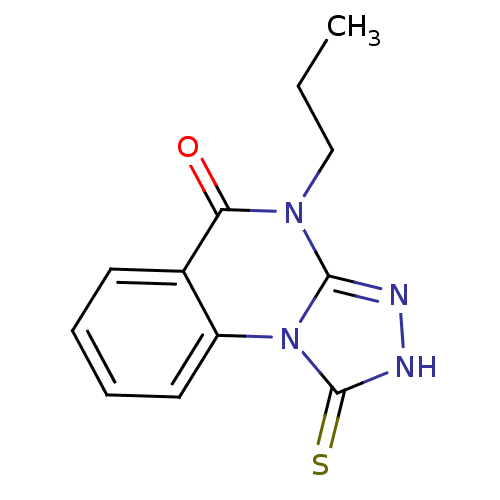
BDBM60899 4-propyl-1-sulfanylidene-2H-[1,2,4]triazolo[4,3-a]quinazolin-5-one::4-propyl-1-thioxo-2H-[1,2,4]triazolo[4,3-a]quinazolin-5-one::MLS001170914::SMR000588314::US20230365568, Compound 21::cid_2423905
SMILES CCCn1c2n[nH]c(=S)n2c2ccccc2c1=O
InChI Key InChIKey=VTEKNXCWZXDPEY-UHFFFAOYSA-N
Data 7 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 60899
Found 7 hits for monomerid = 60899
TargetDNA dC->dU-editing enzyme APOBEC-3G(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 2.87E+3nMT: 2°CAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetDNA dC->dU-editing enzyme APOBEC-3A(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.00E+5nMT: 2°CAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetDNA dC->dU-editing enzyme APOBEC-3G(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 9.90E+4nMT: 2°CAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetDNA dC->dU-editing enzyme APOBEC-3A(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 9.90E+4nMT: 2°CAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Human)
The United States of America,As Represented By The Secretary,Department of Health and Human Services
US Patent
The United States of America,As Represented By The Secretary,Department of Health and Human Services
US Patent
Affinity DataIC50: 1.03E+3nMAssay Description:An ELISA-based PBD-binding inhibition assay was performed essentially as described previously. Briefly, a biotinylated PBIP1 p-T78 peptide (i.e., Bio...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Human)
The United States of America,As Represented By The Secretary,Department of Health and Human Services
US Patent
The United States of America,As Represented By The Secretary,Department of Health and Human Services
US Patent
Affinity DataIC50: 1.03E+3nMAssay Description:An ELISA-based PBD-binding inhibition assay was performed essentially as described previously. Briefly, a biotinylated PBIP1 p-T78 peptide (i.e., Bio...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Human)
The United States of America,As Represented By The Secretary,Department of Health and Human Services
US Patent
The United States of America,As Represented By The Secretary,Department of Health and Human Services
US Patent
Affinity DataIC50: 1.03E+3nMAssay Description:An ELISA-based PBD-binding inhibition assay was performed essentially as described previously. Briefly, a biotinylated PBIP1 p-T78 peptide (i.e., Bio...More data for this Ligand-Target Pair