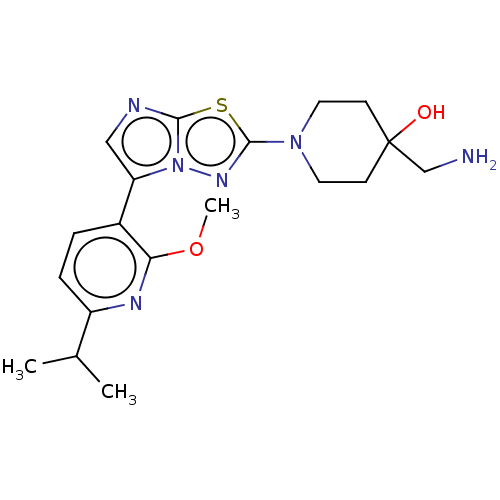
BDBM597562 US11597733, Example 4-0
SMILES COc1nc(ccc1-c1cnc2sc(nn12)N1CCC(O)(CN)CC1)C(C)C
InChI Key InChIKey=JYHSRMKXBAPFST-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 597562
Found 12 hits for monomerid = 597562
Affinity DataKd: >100nMAssay Description:Binding reactions were assembled by combining kinases, liganded affinity beads, and test compounds in 1× binding buffer (20% SeaBlock, 0.17×PBS, 0.05...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Human)
Novartis
US Patent
Novartis
US Patent
Affinity DataKd: >100nMAssay Description:Binding reactions were assembled by combining kinases, liganded affinity beads, and test compounds in 1× binding buffer (20% SeaBlock, 0.17×PBS, 0.05...More data for this Ligand-Target Pair
Affinity DataKd: >100nMAssay Description:Binding reactions were assembled by combining kinases, liganded affinity beads, and test compounds in 1× binding buffer (20% SeaBlock, 0.17×PBS, 0.05...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Human)
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of human PIK3CAMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.30E+4nMAssay Description:Inhibition of hERG by Qpatch-clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of human FLT3More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of human PIM1More data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 8.50E+3nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of CYP2B6 (unknown origin)More data for this Ligand-Target Pair