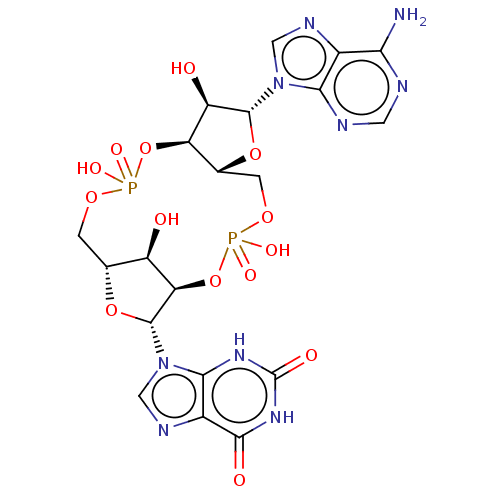
BDBM573885 9-[(5R,7R,8R,12aR,14R,15R,15aS,16R)- 14-(6-amino-9H-purin-9-yl)-2,10,15,16- tetrahydroxy-2,10-dioxidooctahydro-12H- 5,8-methanofuro[3,2-l][1,3,6,9,11,2,10] pentaoxadiphosphacyclotetradecin-7-yl]- 3,9-dihydro-1H-purine-2,6-dione::US11453697, Example 44
SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]2COP(O)(=O)O[C@@H]3[C@H](O)[C@@H](COP(O)(=O)O[C@H]2[C@H]1O)O[C@H]3n1cnc2c1[nH]c(=O)[nH]c2=O
InChI Key InChIKey=DYCTYFRLUMAQAW-UHFFFAOYSA-N
Data 2 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 573885
Found 2 hits for monomerid = 573885
TargetStimulator of interferon genes protein [1-379,R71H,G230A,H232R,R293Q](Human)
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataEC50: 17.1nMAssay Description:The compounds were serially titrated by the Hamilton STARPlus CORE in a 96-well plate (Greiner, #651201) using a 1:3 ten-point dose response format. ...More data for this Ligand-Target Pair