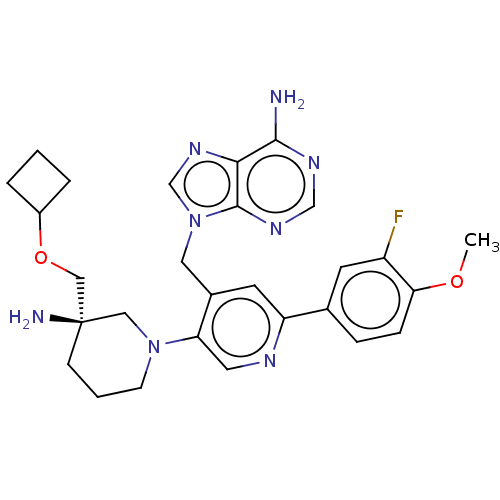
BDBM567831 US11420970, Example 32
SMILES COc1ccc(cc1F)-c1cc(Cn2cnc3c(N)ncnc23)c(cn1)N1CCC[C@](N)(COC2CCC2)C1
InChI Key InChIKey=WLGCCCLQJAMXCP-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 567831
Found 3 hits for monomerid = 567831
Affinity DataIC50: 5.5nMAssay Description:This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-36 specific(Human)
University of Texas Medical Branch (UTMB)
Curated by ChEMBL
University of Texas Medical Branch (UTMB)
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibition of human recombinant NSD1 incubated for 2 hrs by AlphaLISA methodMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase NSD3(Human)
University of Texas Medical Branch (UTMB)
Curated by ChEMBL
University of Texas Medical Branch (UTMB)
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of NSD3 degradation in human KMS-11 cells assessed as reduction in H3K36me2 levels by FRET assayMore data for this Ligand-Target Pair