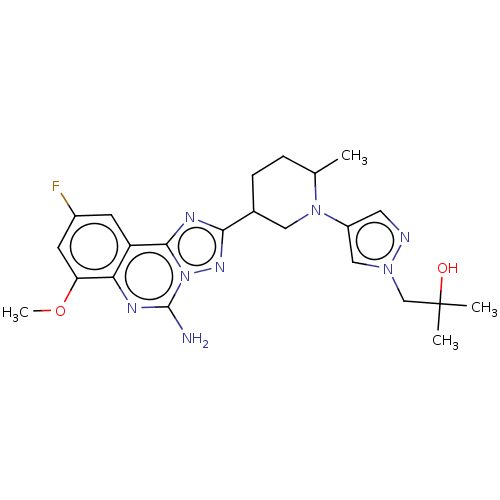
BDBM551572 1-(4-((2R or 2S,5R or 5S)-5-(5-amino-9-fluoro-7-methoxy- [1,2,4]triazolo[1,5-c]quinazolin-2-yl)-2-methylpiperidin-1- yl)-1H-pyrazol-1-yl)-2-methylpropan-2-ol::US11312719, Example 118::US11312719, Example 119::US11312719, Example 120::US11312719, Example 121
SMILES COc1cc(F)cc2c3nc(nn3c(N)nc12)C1CCC(C)N(C1)c1cnn(CC(C)(C)O)c1
InChI Key InChIKey=ZGHVEDRQDAFOKF-UHFFFAOYSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 551572
Found 8 hits for monomerid = 551572
Affinity DataIC50: 1.90nMAssay Description:148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were...More data for this Ligand-Target Pair
Affinity DataIC50: 387nMAssay Description:The reported affinity of the compounds of the invention for the human A2b adenosine receptor was determined experimentally using a radioligand filtra...More data for this Ligand-Target Pair
Affinity DataIC50: 51.6nMAssay Description:148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:The reported affinity of the compounds of the invention for the human A2b adenosine receptor was determined experimentally using a radioligand filtra...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were...More data for this Ligand-Target Pair
Affinity DataIC50: 7.80nMAssay Description:The reported affinity of the compounds of the invention for the human A2b adenosine receptor was determined experimentally using a radioligand filtra...More data for this Ligand-Target Pair
Affinity DataIC50: 55.4nMAssay Description:148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were...More data for this Ligand-Target Pair
Affinity DataIC50: 5.34E+3nMAssay Description:The reported affinity of the compounds of the invention for the human A2b adenosine receptor was determined experimentally using a radioligand filtra...More data for this Ligand-Target Pair