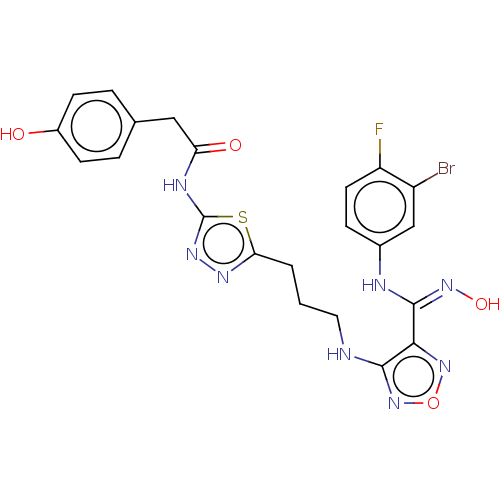
BDBM50633100 CHEMBL5412818
SMILES O\N=C(\Nc1ccc(F)c(Br)c1)c1nonc1NCCCc1nnc(NC(=O)Cc2ccc(O)cc2)s1
InChI Key InChIKey=UEYJCFRMVBBVMR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50633100
Found 12 hits for monomerid = 50633100
Affinity DataEC50: 4.56E+4nMAssay Description:Inhibition of IDO1 in human HeLa cells using L-Tryptophan as substrate incubated for 24 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 2.32E+6nMAssay Description:Inhibition of IDO1 in mouse CT26 cells using L-Tryptophan as substrate incubated for 24 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 3.41E+4nMAssay Description:Inhibition of IDO1 in human HCT-116 cells using L-Tryptophan as substrate incubated for 24 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 1.31E+4nMAssay Description:Inhibition of TrxR1 in human HeLa cells incubated for 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 3.14E+3nMAssay Description:Inhibition of TrxR1 in human HCT-116 cells incubated for 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 2.57E+4nMAssay Description:Inhibition of TrxR1 in mouse CT26 cells incubated for 24 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.42E+4nMAssay Description:Inhibition of TDO (unknown origin) using L-Trp as substrate incubated for 75 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.22E+4nMAssay Description:Inhibition of IDO2 (unknown origin) using D-Trp as substrate incubated for 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+5nMAssay Description:Inhibition of TrxR3 (unknown origin) incubated for 1 hr in presence of NADPH by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+5nMAssay Description:Inhibition of TrxR2 (unknown origin) incubated for 1 hr in presence of NADPH by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of recombinant rat TrxR1 incubated for 1 hr in presence of NADPH by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of recombinant human IDO1 using L-Trp as substrate incubated for 1 hrMore data for this Ligand-Target Pair