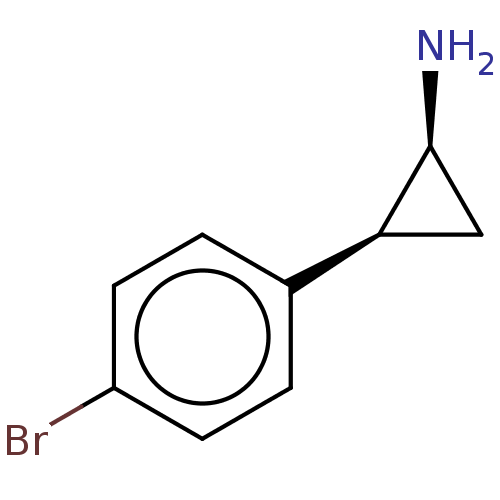
BDBM50616050 CHEMBL5439284
SMILES N[C@H]1C[C@H]1c1ccc(Br)cc1
InChI Key InChIKey=XRLLHILOPOQXRW-UHFFFAOYSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50616050
Found 3 hits for monomerid = 50616050
Affinity DataKi: 2.30E+3nMAssay Description:Inhibition of human recombinant MAO-B expressed in Pichia pastoris assessed as inhibition constant using benzylamine as substrate by peroxidase-coupl...More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+4nMAssay Description:Inhibition of human recombinant LSD1/CoREST expressed in Escherichia coli using Lys4 monomethylated histone H3 peptide as substrate assessed as inhib...More data for this Ligand-Target Pair
Affinity DataKi: 8.20E+4nMAssay Description:Inhibition of mouse recombinant LSD2 expressed in Escherichia coli using Lys4 dimethylated histone H3 peptide assessed as inhibition constant by pero...More data for this Ligand-Target Pair