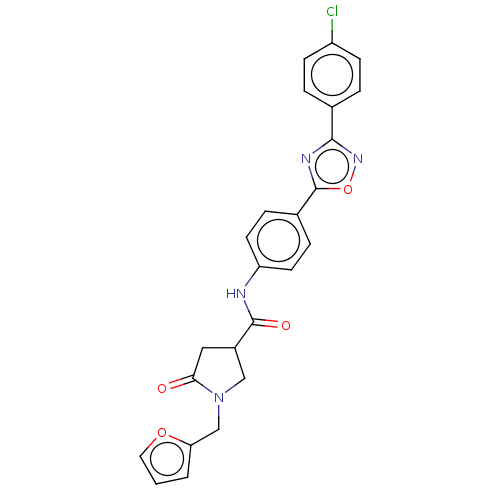
BDBM50606009 CHEMBL5207128
SMILES Clc1ccc(cc1)-c1noc(n1)-c1ccc(NC(=O)C2CN(Cc3ccco3)C(=O)C2)cc1
InChI Key InChIKey=LTOAWFRZXVPHAJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50606009
Found 4 hits for monomerid = 50606009
Affinity DataIC50: 3.08E+3nMAssay Description:Antagonist activity at human RXFP3 expressed in CHO-K1 cells assessed as forskolin-induced cAMP accumulation measured after 30 mins in presence of re...More data for this Ligand-Target Pair
Affinity DataIC50: 4.52E+3nMAssay Description:Antagonist activity at human RXFP3 expressed in CHO-K1 cells assessed as [35S]GTPgammaS binding incubated for 1 hr by [35S]GTP-gammaS binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.39E+4nMAssay Description:Antagonist activity at human RXFP3 expressed in CHO-K1 cells assessed as inhibition of relaxin-3 stimulated ERK1/2 phosphorylation preincubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 3.20E+3nMAssay Description:Inhibition of sigma-2 receptor (unknown origin)More data for this Ligand-Target Pair