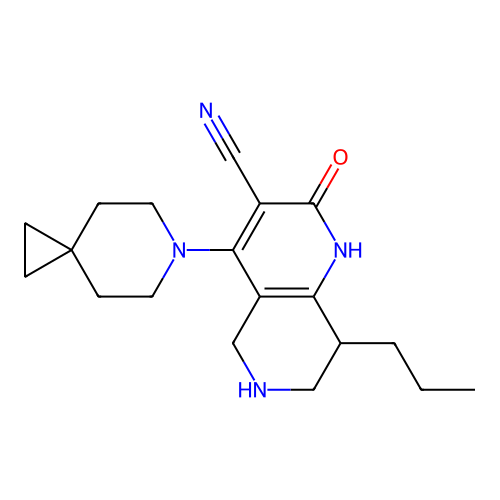
BDBM50602398 CHEMBL5198935
SMILES CCCC1CNCc2c1[nH]c(=O)c(C#N)c2N1CCC2(CC2)CC1
InChI Key InChIKey=RVOZYMPVGIVNJX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50602398
Found 4 hits for monomerid = 50602398
Affinity DataIC50: 15nMAssay Description:Inhibition of full length human PDE9A expressed in Sf9 insect cells using [3H]-cGMP as substrate incubated for 60 mins by microbeta scintillation cou...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of full length human PDE9A expressed in Sf9 insect cells using [3H]-cGMP as substrate incubated for 60 mins by microbeta scintillation cou...More data for this Ligand-Target Pair
Affinity DataEC50: 1.00E+3nMAssay Description:Inhibition of mouse PDE9A expressed in recombinant CHO cells coexpressing soluble guanylate cyclase incubated for 6 mins by [3H]-cGMP scintillation p...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate by LC-MS/MS analysisMore data for this Ligand-Target Pair