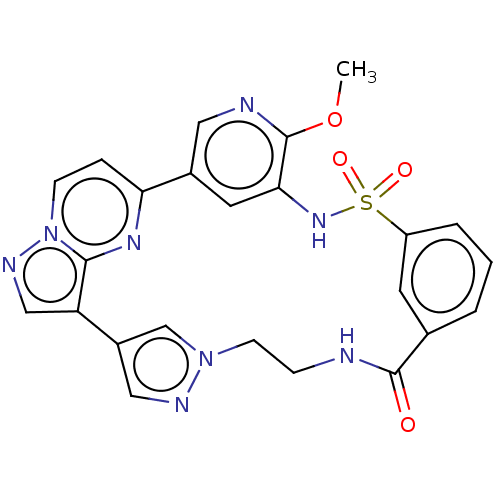
BDBM50570245 CHEMBL4875243
SMILES COc1ncc-2cc1NS(=O)(=O)c1cccc(c1)C(=O)NCCn1cc(cn1)-c1cnn3ccc-2nc13
InChI Key InChIKey=FWPPYFMXRQXAML-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50570245
Found 3 hits for monomerid = 50570245
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Human)
Spanish National Cancer Research Centre (Cnio)
Curated by ChEMBL
Spanish National Cancer Research Centre (Cnio)
Curated by ChEMBL
Affinity DataIC50: 7.70nMAssay Description:Inhibition of human full length p110alpha (1 to 1068 residues) expressed in baculovirus-infected Sf21 cells by ADP-Hunter Plus assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase mTOR(Human)
Spanish National Cancer Research Centre (Cnio)
Curated by ChEMBL
Spanish National Cancer Research Centre (Cnio)
Curated by ChEMBL
Affinity DataIC50: 66nMAssay Description:Inhibition of recombinant human GST tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system measured by LanthaScreen assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Spanish National Cancer Research Centre (Cnio)
Curated by ChEMBL
Spanish National Cancer Research Centre (Cnio)
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG by fluorescence polarization assayMore data for this Ligand-Target Pair