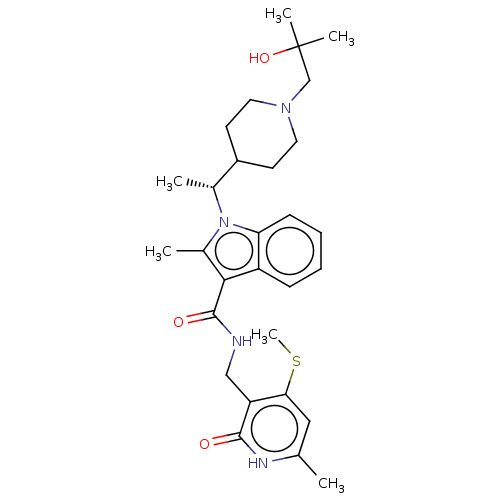
BDBM50541902 CHEMBL4635809::US11459315, Example 11
SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CC(C)(C)O)CC2)c2ccccc12
InChI Key InChIKey=OFNLBVMSPMQAEB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50541902
Found 3 hits for monomerid = 50541902
Affinity DataIC50: 0.0670nMAssay Description:Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA...More data for this Ligand-Target Pair
Affinity DataEC50: 26nMAssay Description:Inhibition of EZH2 in human HeLa cells assessed as inhibition of trimethylation of H3K27 after 72 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame...More data for this Ligand-Target Pair