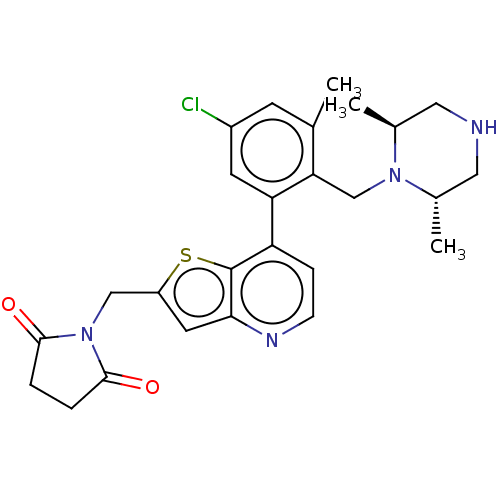
BDBM50538575 CHEMBL4638458
SMILES C[C@H]1CNC[C@H](C)N1Cc1c(C)cc(Cl)cc1-c1ccnc2cc(CN3C(=O)CCC3=O)sc12
InChI Key InChIKey=ZPKJNNBGFQUARW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50538575
Found 3 hits for monomerid = 50538575
Affinity DataIC50: 0.320nMAssay Description:Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad...More data for this Ligand-Target Pair
Affinity DataEC50: 60nMAssay Description:Inhibition of USP7 in human RKO cells transfected with p53 luciferase reporter vector assessed as increase in p53-dependent luciferase activity measu...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair