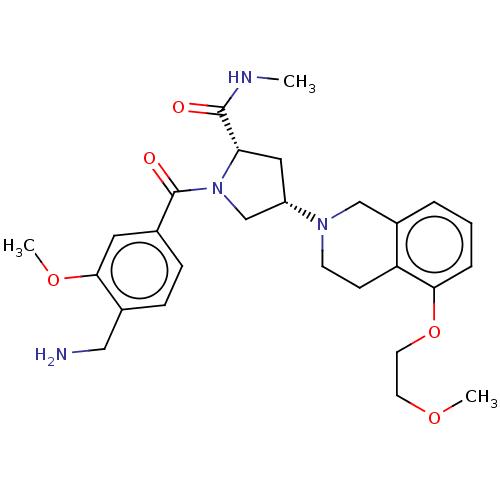
BDBM50533254 CHEMBL4550207
SMILES CNC(=O)[C@@H]1C[C@@H](CN1C(=O)c1ccc(CN)c(OC)c1)N1CCc2c(C1)cccc2OCCOC
InChI Key InChIKey=OBAPCSRNRUUGDW-UHFFFAOYSA-N
Data 12 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50533254
Found 12 hits for monomerid = 50533254
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human f10aMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human f10aMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair