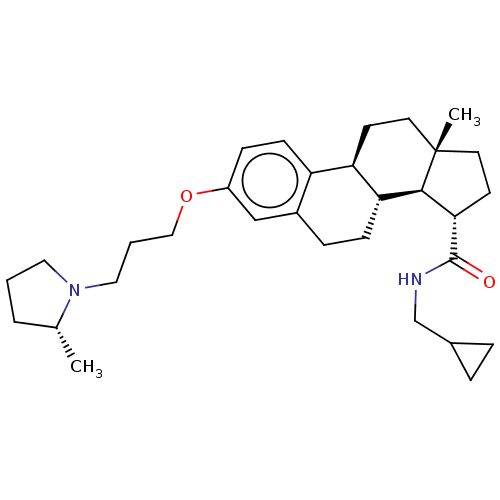
BDBM50525822 CHEMBL4515933
SMILES [H][C@@]12[C@H](CC[C@@]1(C)CC[C@]1([H])c3ccc(OCCCN4CCC[C@H]4C)cc3CC[C@@]21[H])C(=O)NCC1CC1
InChI Key InChIKey=XVTLHDIVQKIZDR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50525822
Found 3 hits for monomerid = 50525822
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Chemical Works of Gedeon Richter
Curated by ChEMBL
Chemical Works of Gedeon Richter
Curated by ChEMBL
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Displacement of [3H]methyl- N-alpha methylhistamine dihydrochloride from human recombinant H3 receptor expressed in CHOK1 cells measured after 30 min...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Displacement of [3H]methyl- N-alpha methylhistamine dihydrochloride from Sprague-Dawley rat cerebral cortex H3 receptor measured after 30 mins by sci...More data for this Ligand-Target Pair