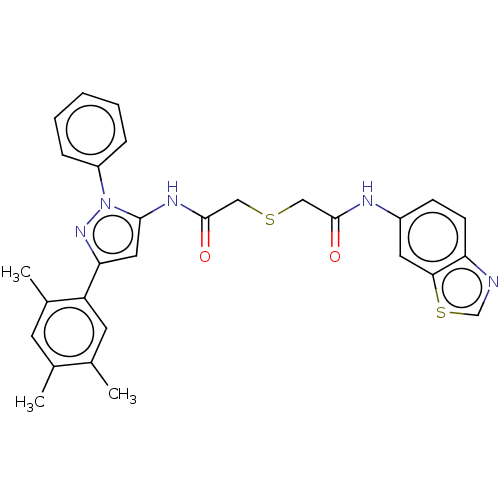
BDBM50514158 CHEMBL4526913
SMILES Cc1cc(C)c(cc1C)-c1cc(NC(=O)CSCC(=O)Nc2ccc3ncsc3c2)n(n1)-c1ccccc1
InChI Key InChIKey=UHBHMCUEAHAUJJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50514158
Found 5 hits for monomerid = 50514158
Affinity DataIC50: 0.780nMAssay Description:Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici...More data for this Ligand-Target Pair
Affinity DataIC50: 172nMAssay Description:Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici...More data for this Ligand-Target Pair
Affinity DataKd: 2.56E+4nMAssay Description:Inhibition of spin-labelled adenine nucleotide interaction with human P-gp expressed in Escherichia coli BL21 (DE3) assessed as apparent dissociation...More data for this Ligand-Target Pair