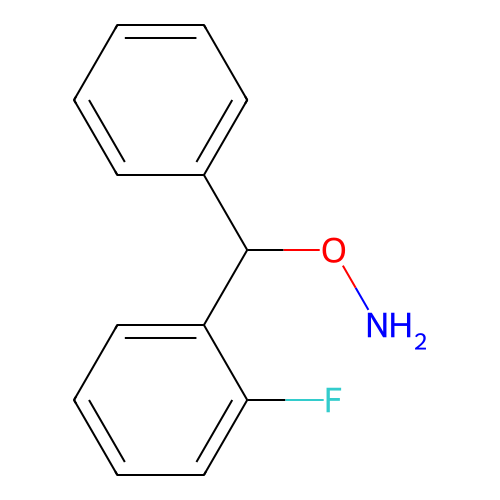
BDBM50507278 CHEMBL4446245
SMILES NOC(c1ccccc1)c1ccccc1F
InChI Key InChIKey=OJDSVLWIEBXHPC-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50507278
Found 4 hits for monomerid = 50507278
Affinity DataIC50: 1.26E+3nMAssay Description:Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate after 20 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.92E+3nMAssay Description:Inhibition of human TDO expressed in human T-REx cells using L-tryptophan as substrate after 20 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.05E+4nMAssay Description:Inhibition of mouse IDO2 expressed in human T-REx cells using L-tryptophan as substrate after 20 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of IDO1 (unknown origin) using L-tryptophan as substrate by spectrophotometric methodMore data for this Ligand-Target Pair