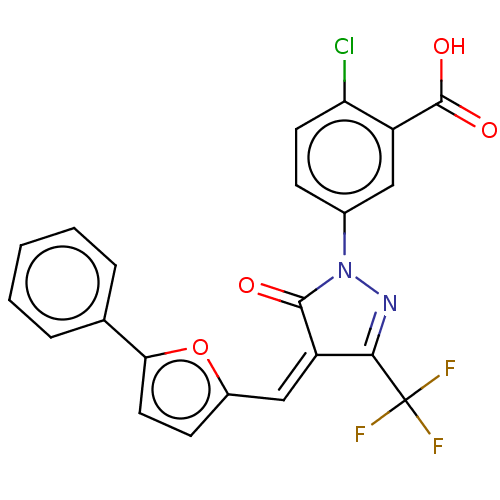
BDBM50505175 CHEMBL4303182
SMILES OC(=O)c1cc(ccc1Cl)N1N=C(\C(=C\c2ccc(o2)-c2ccccc2)C1=O)C(F)(F)F
InChI Key InChIKey=RSLFQCNAOMQAIH-UHFFFAOYSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50505175
Found 5 hits for monomerid = 50505175
Affinity DataIC50: 4.16E+3nMAssay Description:Inhibition of recombinant human LSD1 using ARTK(diMethyl)QTARKSTGGKAPRKQLAPRKQLA as substrate measured after 30 mins by ADHP/horseradish peroxidase c...More data for this Ligand-Target Pair
Affinity DataIC50: 2.21E+4nMAssay Description:Inhibition of recombinant human Ero1Lalpha C104A/C131A/C166A triple mutant (22 to 468 residues) expressed in Escherichia coli BL21(DE3)-RIL using hum...More data for this Ligand-Target Pair
Affinity DataIC50: 7.91E+3nMAssay Description:Inhibition of MAOA (unknown origin) using kynuramine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.27E+4nMAssay Description:Inhibition of recombinant human Ero1Lalpha C104A/C131A/C166A triple mutant (22 to 468 residues) expressed in Escherichia coli BL21(DE3)-RIL measured ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.06E+4nMAssay Description:Inhibition of MAOB (unknown origin) using kynuramine as substrateMore data for this Ligand-Target Pair