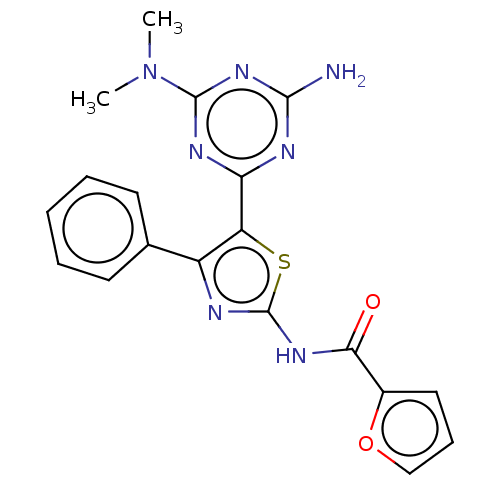
BDBM50492059 CHEMBL2391837
SMILES CN(C)c1nc(N)nc(n1)-c1sc(NC(=O)c2ccco2)nc1-c1ccccc1
InChI Key InChIKey=DDDMPSVRAPVZGB-UHFFFAOYSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50492059
Found 4 hits for monomerid = 50492059
TargetAdenosine receptor A2a(Human)
B.V. Patel Pharmaceutical Education and Research Development
Curated by ChEMBL
B.V. Patel Pharmaceutical Education and Research Development
Curated by ChEMBL
Affinity DataKi: 179nMAssay Description:Displacement of [3H]NECA from human adenosine A2A receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A1(Human)
B.V. Patel Pharmaceutical Education and Research Development
Curated by ChEMBL
B.V. Patel Pharmaceutical Education and Research Development
Curated by ChEMBL
Affinity DataKi: 536nMAssay Description:Displacement of [3H]CCPA from human adenosine A1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Human)
B.V. Patel Pharmaceutical Education and Research Development
Curated by ChEMBL
B.V. Patel Pharmaceutical Education and Research Development
Curated by ChEMBL
Affinity DataKi: 918nMAssay Description:Displacement of [3H]HEMADO from human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A2b(Human)
B.V. Patel Pharmaceutical Education and Research Development
Curated by ChEMBL
B.V. Patel Pharmaceutical Education and Research Development
Curated by ChEMBL
Affinity DataKi: 8.00E+3nMAssay Description:Antagonist activity at human adenosine A2B receptor expressed in CHO cells assessed as inhibition of NECA-stimulated adenylyl cyclase activityMore data for this Ligand-Target Pair